Pharmacopuncture or herbal acupuncture is a totally different modality of treatment from the traditional methods used in Korean medicine. Its therapy was derived by combining two traditional therapeutic methods, herbal medicine and acupuncture therapy. Pharmacopuncture treatment is performed by injecting small amounts of herbal medicinal materials at acupuncture points or affected areas in order to achieve the effects of both herb medicine and acupuncture [1]. In the Korean clinical environment, pharmacopuncture is frequently used on a daily basis. Nowadays, its safety and efficacy are important issues.
Panax ginseng (Korean ginseng) has been used as a traditional medicine for boosting Qi energy and tonifying the spleen and lungs [2]. It has also been used as a traditional medicine for the treatment of cancer and has been shown to inhibit tumor cell proliferation and tumor growth, to induce differentiation and apoptosis, and to inhibit cancer cell invasion [3-6]. The major important components of Panax ginseng are saponin glycosides, which are known as the ginsenosides, a group of steroidal saponins. Until now, over 50 ginsenosides have been isolated from ginseng saponins [7-9]. Ginsenosides are characterized by a steroid like skeleton consisting of 4 trans rings, with modifications that depend on the type (e.g., glucose, maltose, and fructose) and the number of sugar moieties, as well as the sites of attachment of the hydroxyl group (e.g., C-3, C-6, or C-20). Based on their chemical structural characteristics, ginseng saponins can be divided into protopanaxadiol and protopanaxatriol groups, except for ginsenoside Ro, which is derived from an oleanolic group. In the protopanaxadiol group, sugars are attached to the
Generally, ginseng is a representative herb that increases immunity, reduces fatigue, increases blood circulation, increases memory, and helps with anti-oxidation. Also, special ginseng that consists of low molecular weight ginsenosides (ginsenosides Rg3, Rh1, Rh2, Compound K, etc.) have been synthesized by using an enzyme treated technique. This increases absorption in patients lacking the body enzymes necessary to degrade ginseng, thus yielding superior efficacy [10, 11]. However, low molecular weight ginsenosides in ginseng are not soluble in water, so we must use homogenized technology for make a water-soluble ginseng pharmacopuncture. Because we want to know its safety
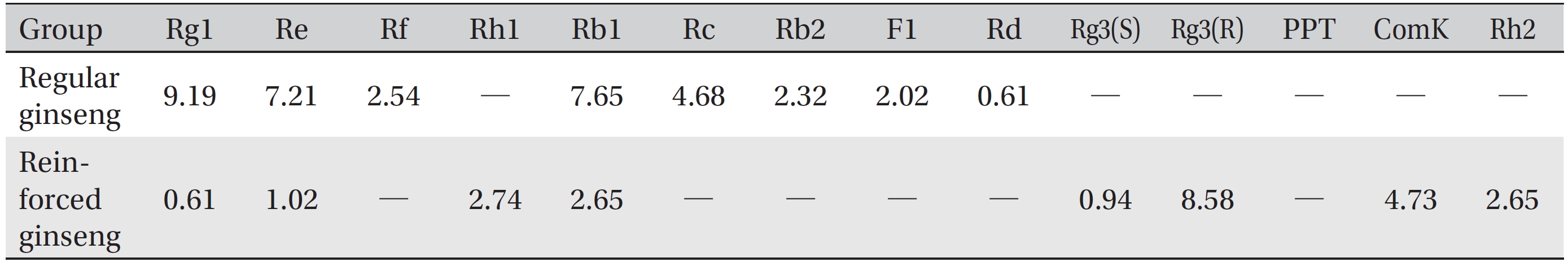
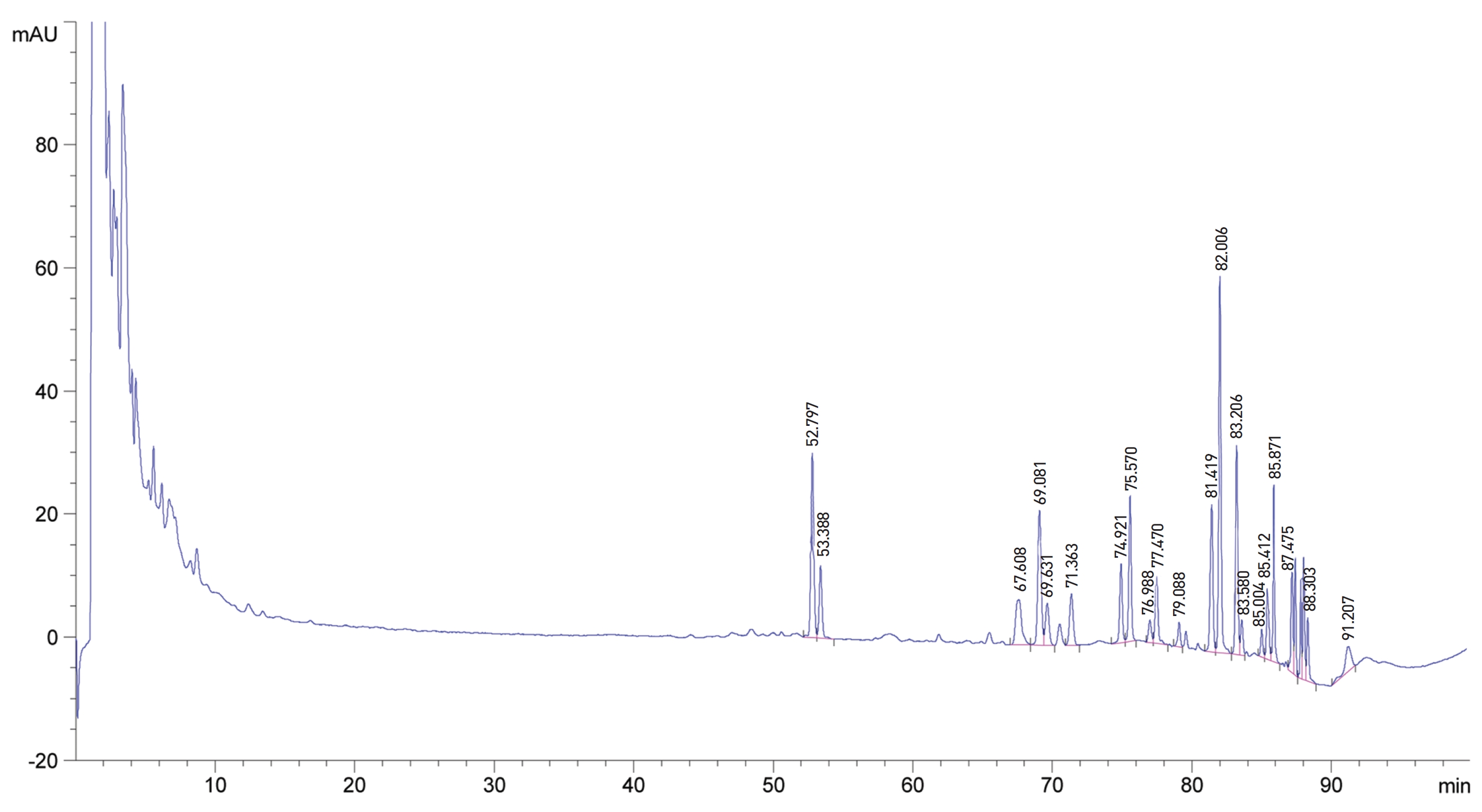
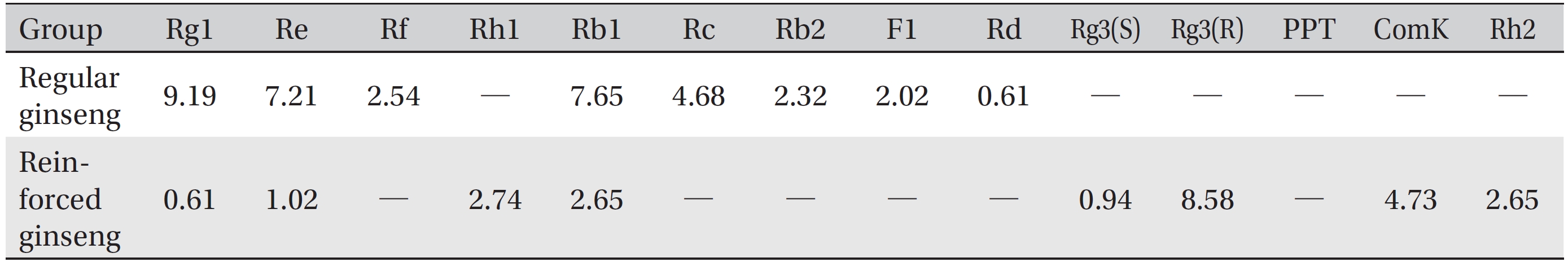
The water soluble ginseng pharmacopuncture was prepared in a sterile room at the Korean Pharmacopuncture Institute (Korea-Good Manufacturing Practice, K-GMP). After the mixing process with pure water had been completed, the pH was controlled to between 7.0 and 7.5; then, NaCl was added to make a 0.9% isotonic solution. The completed extract was stored in a refrigerator (2.1 ─ 6.6°C). A high performance liquid chromatography (HPLC) analysis was performed to determine the changes in the chemical constituents in ginseng saponin following the enzyme treatment. HPLC results showed the appearance of new peaks (Rh1, Rg3, protopanaxtriol (PPT), Compound K, and Rh2), indicative of ginseng saponin metabolites (Fig. 1, Table 1).
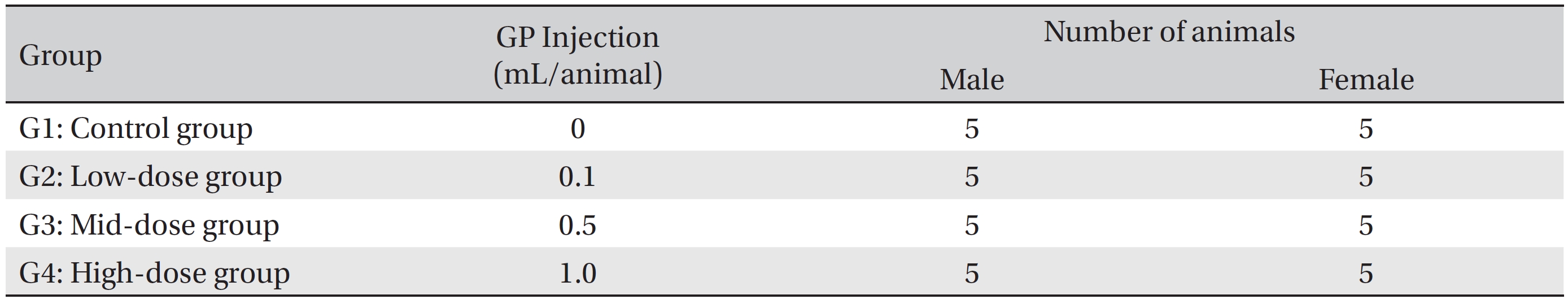
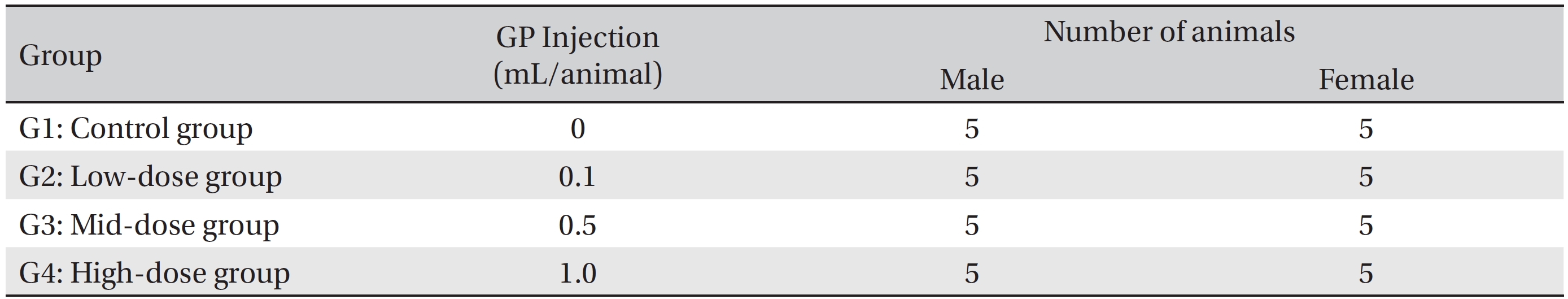
The animals used in this study were 6 week old Sprague-Dawley (SD) rats (Orientbio Inc., Korea). The rats were received at an age of 5 weeks, and they were kept for 1 week at room temperature. The mean weights of the rats were 189.5 ─ 209.2 g (male) and 145.1 ─ 167.6 g (female) at the time of injection. For all animals, a visual inspection was conducted; all animals were weighed using a CP3202S system (Sartorius, Germany). During the 7 days of acclimatization, the general symptoms of the rats were observed once a day. The weights of the rats were recorded on the last day of acclimatization. No abnormalities were found. The temperature of the laboratory was 21.0 ─ 23.2°C, and the humidity was 40.9% ─ 59.4%. Enough food (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918C) and ultra violet (UV)-filtered water were provided. The lights were on for 12 hours/day (from 7 am to 7 pm). Groupings were done after 7 days of acclimatization. Animals were selected if their weights were close to the mean weight. In total, 20 male rats and 20 female rats were selected. The animals were randomly distributed into 4 groups (5 male and 5 female rats per group, Table 2).
The administration route was intramuscular because of clinical considerations, and the administered volume was 1.0 mL/animal of normal saline in the control group and high dose group, 0.1 mL/animal in low dose group, 0.5 mL/ animal in mid dose group. The syringe used in the experiment was 1 mL disposable 26G syringe. For the low dose group and mid dose group, the rats were administrated on the Lt. thigh, single injection. But for the control group and high dose group, the rats on the both thigh, 0.5 mL on each thigh.
The expected volume of GP administered in clinical use is 1.0 mL per treatment. No death occurred in a pilot test in which 1.0 mL of GP was injected into each male and female rat. In this study 1.0 mL/animal was set as a high dose, and 0.5 mL and 0.1 mL were set as the mid and the low doses, respectively. In the control group, 1.0 mL of normal saline solution was administered. This study was conducted under the approval of the Institutional Animal Ethic Committee of Biotoxtech.
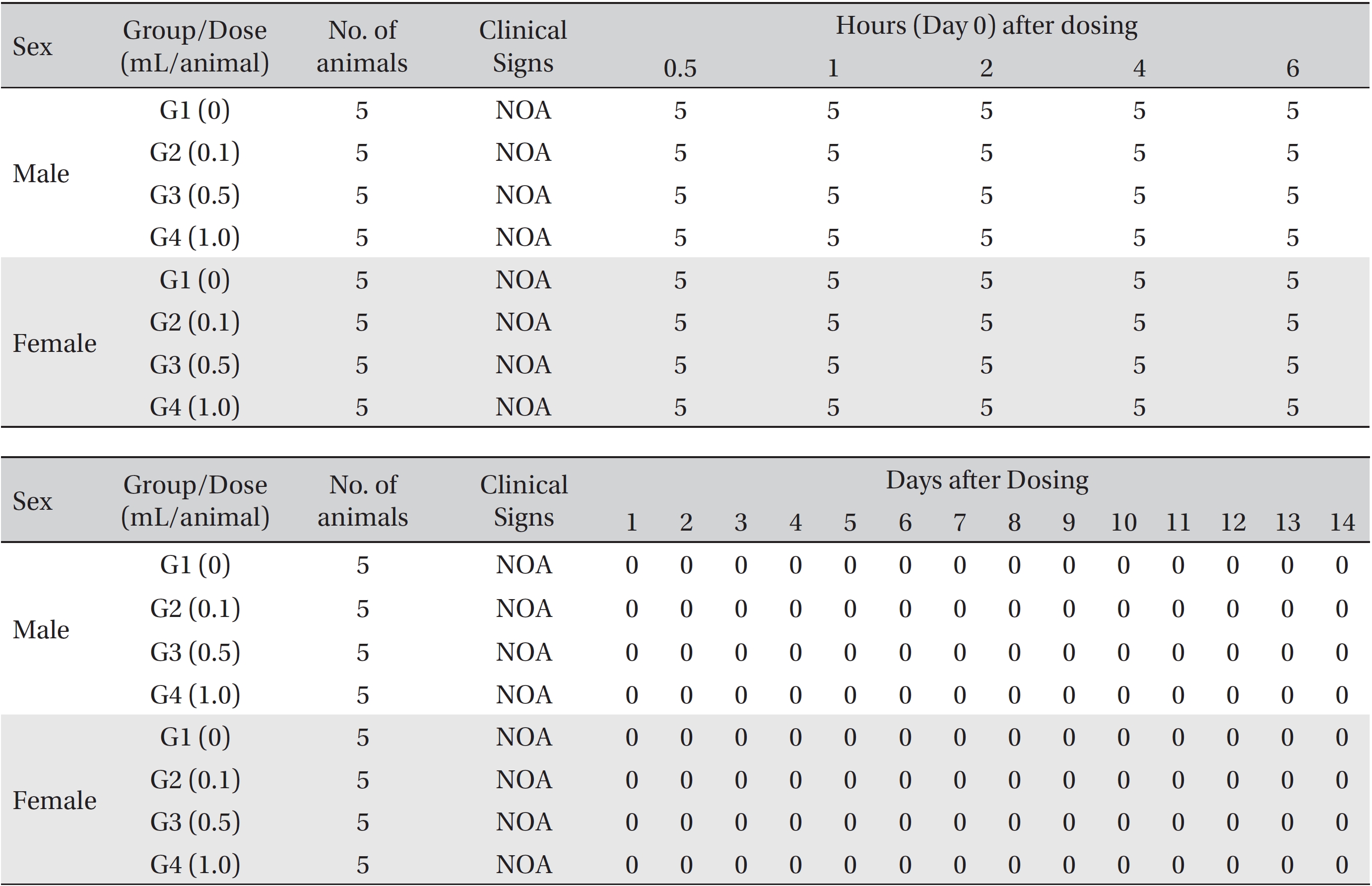
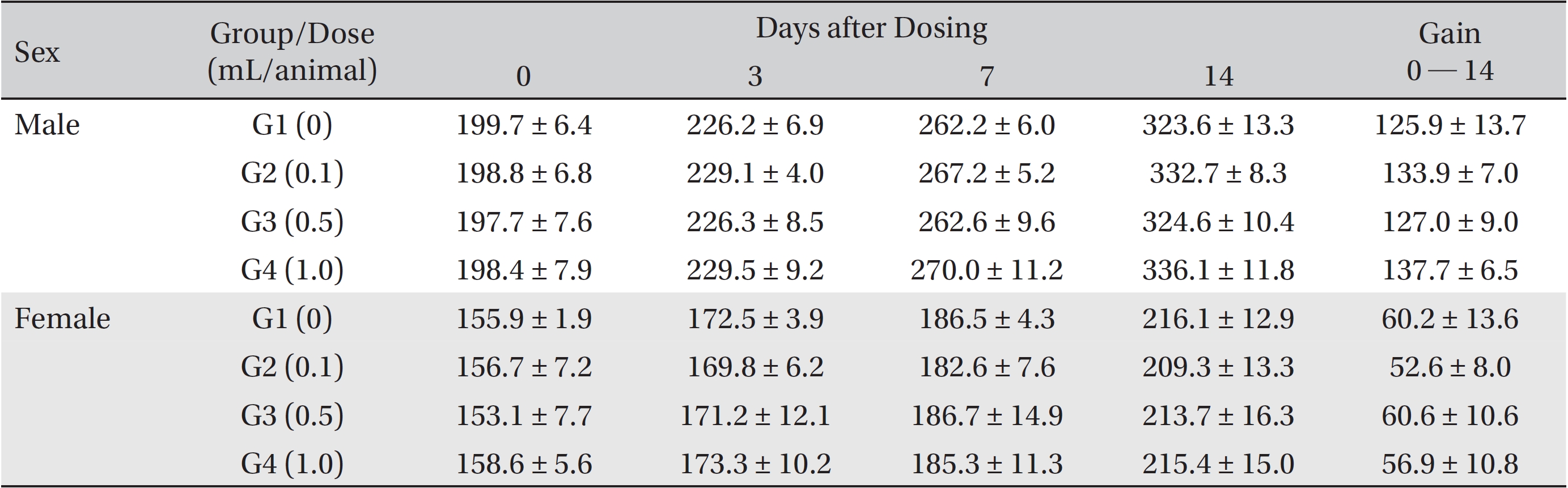
From the 1st day to the 14th day of treatment, the general symptoms were examined once a day. On the day of injection (day 0), the general symptoms (toxicological effects, manifestation time, recovery time, etc.), as well as mortality, were examined at 30 minutes and 1, 2, 3, and 4 hours after injection. Body weights were measured immediately before treatment and at 3, 7 and 14 days after treatment.
After the rats had fasted for more than 18 hours, they were anesthetized by using
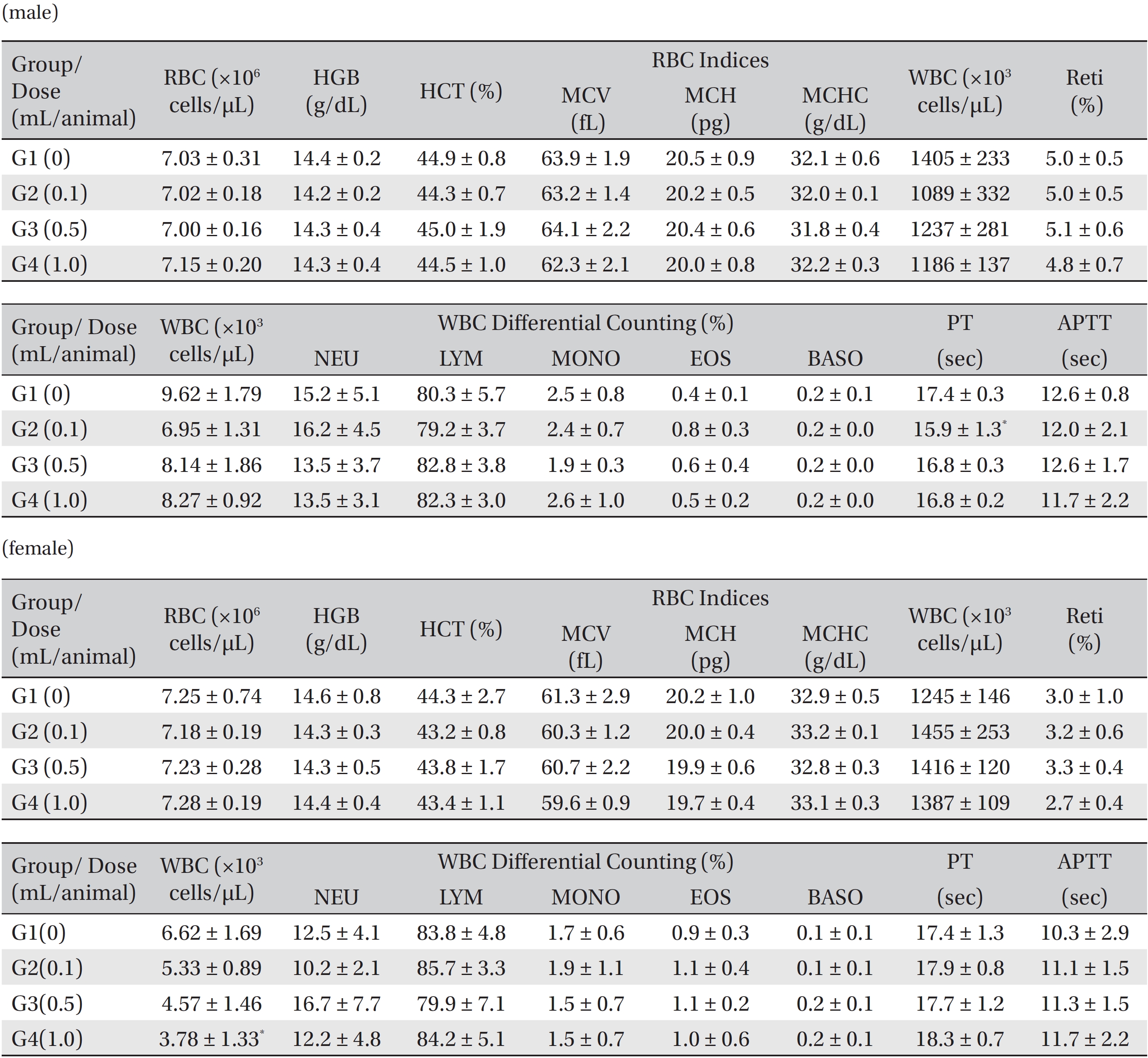
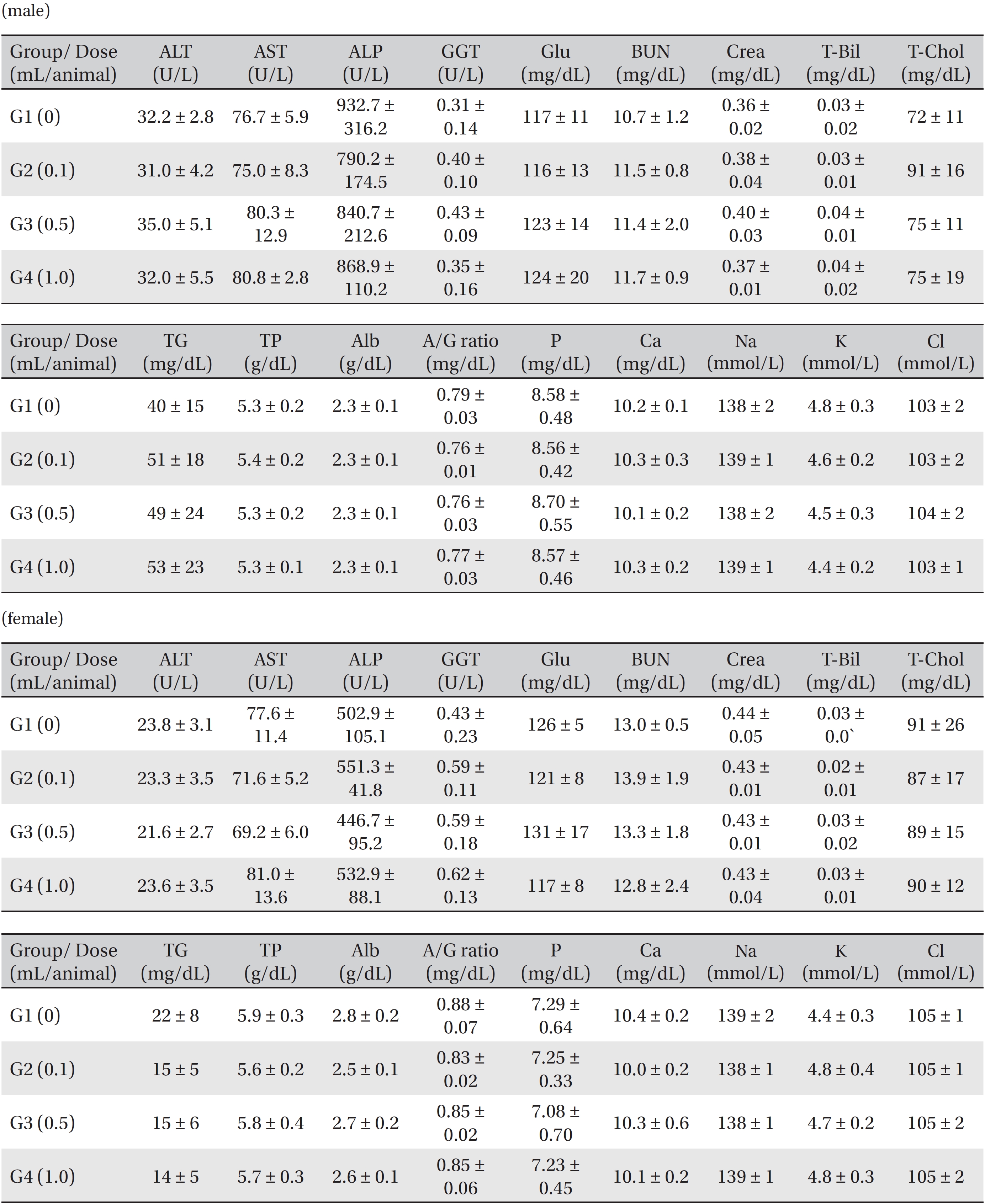
Blood taken from the abdominal aorta was used in the blood biochemical test. The results were measured by using an automatic analyzer (7180, HITACHI, Japan) and an electrolyte analyzer (AVL9181, Roche, Germany). The items measured were alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), blood urea nitrogen (BUN), creatinine (Crea), total bilirubin (T-Bili), total protein (TP), albumin (Alb), albumin/globulin (A/G) ratio, total cholesterol (T-Chol), triglyceride (TG), phosphate (P), glucose (Glu), calcium (Ca), chloride (Cl) and potassium (K).
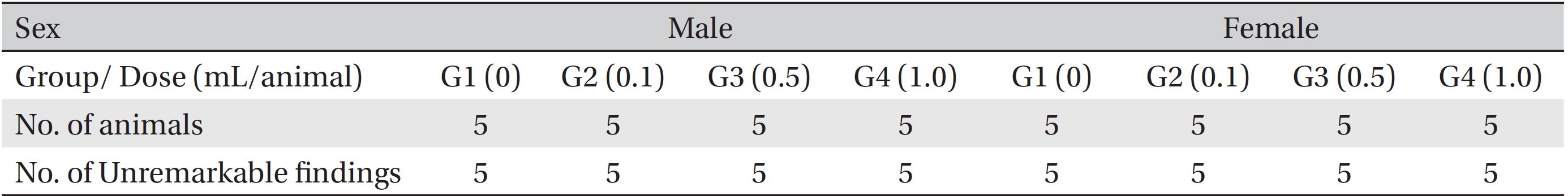
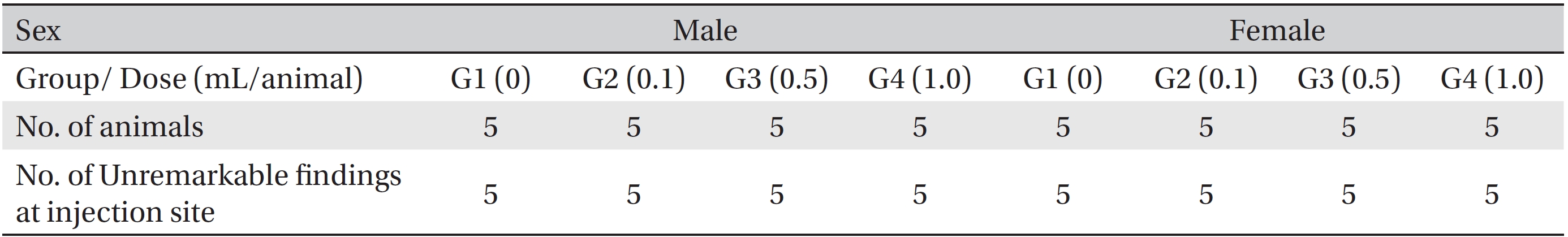
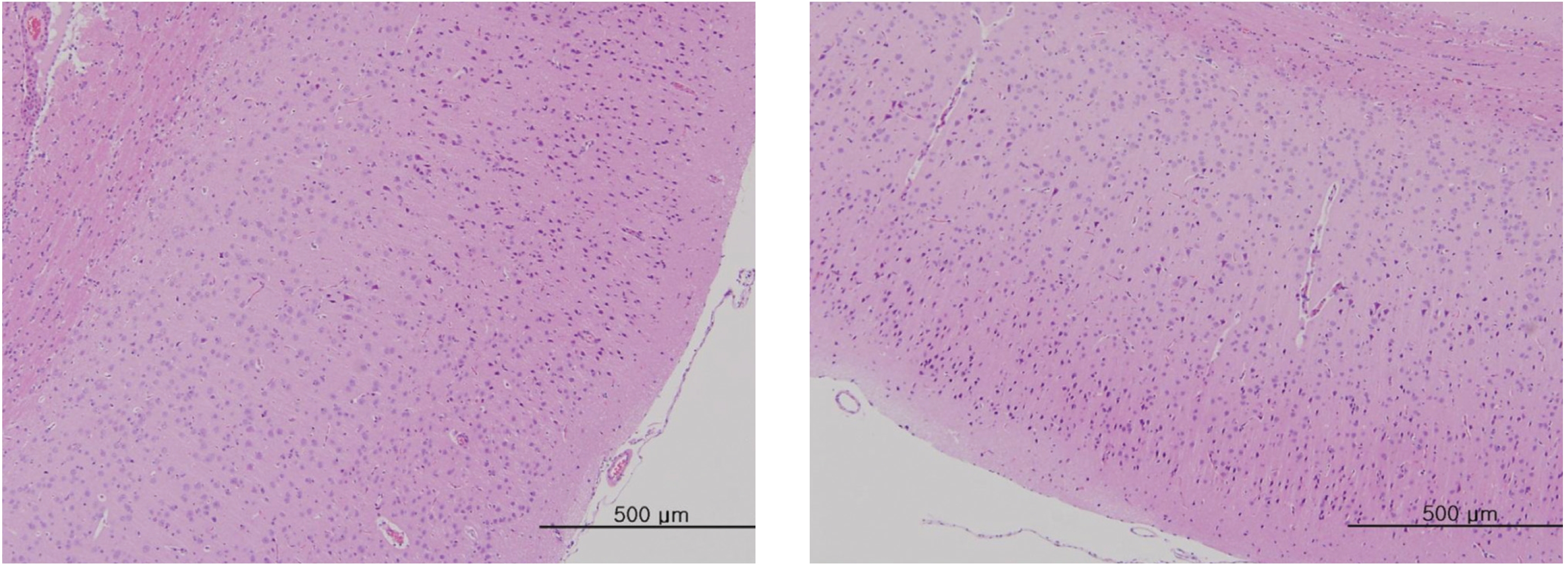
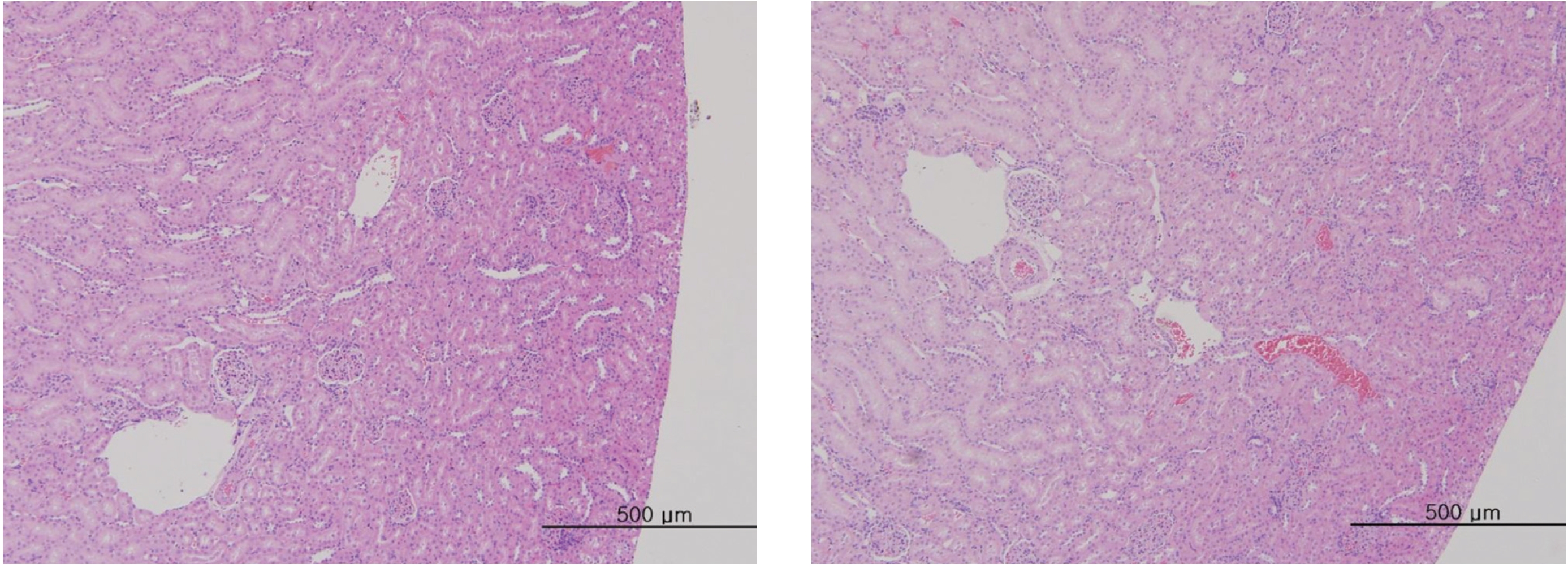
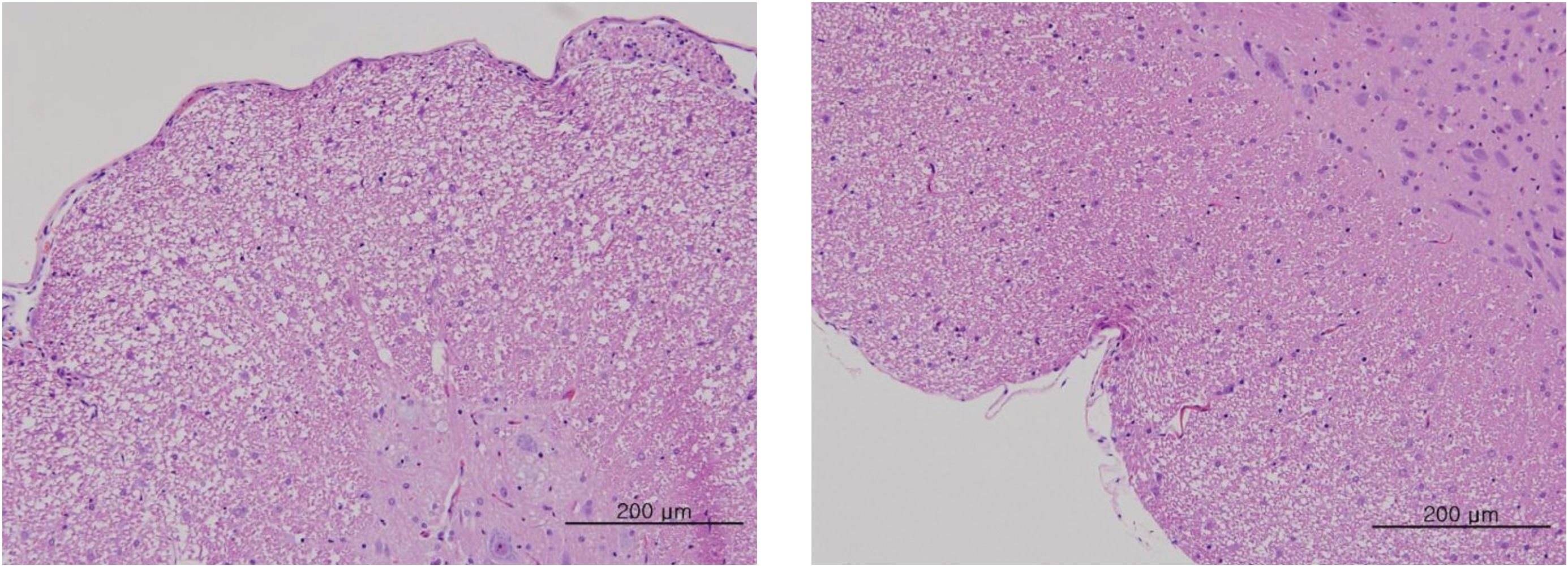
After the termination of all observations, organs and tissues of all surviving animals were visually inspected and were examined under a microscope after they had been stabilized using 10% neutral buffered formalin. For the injection site, tissue slices were stained with hematoxylin & eosin (H&E).
The body weights and the results from the hematologic examinations and the blood biochemical tests were analyzed by using statistical analysis system (SAS) software (version 9.3, SAS Institute Inc., U.S.A.). The Bartlett test was conducted to evaluate the homogeneity of the variance and the significance. The significance level was 0.05. The one-way analysis of variance (ANOVA) test was conducted, and when homogeneity of the variance was recognized, Dunnett’s
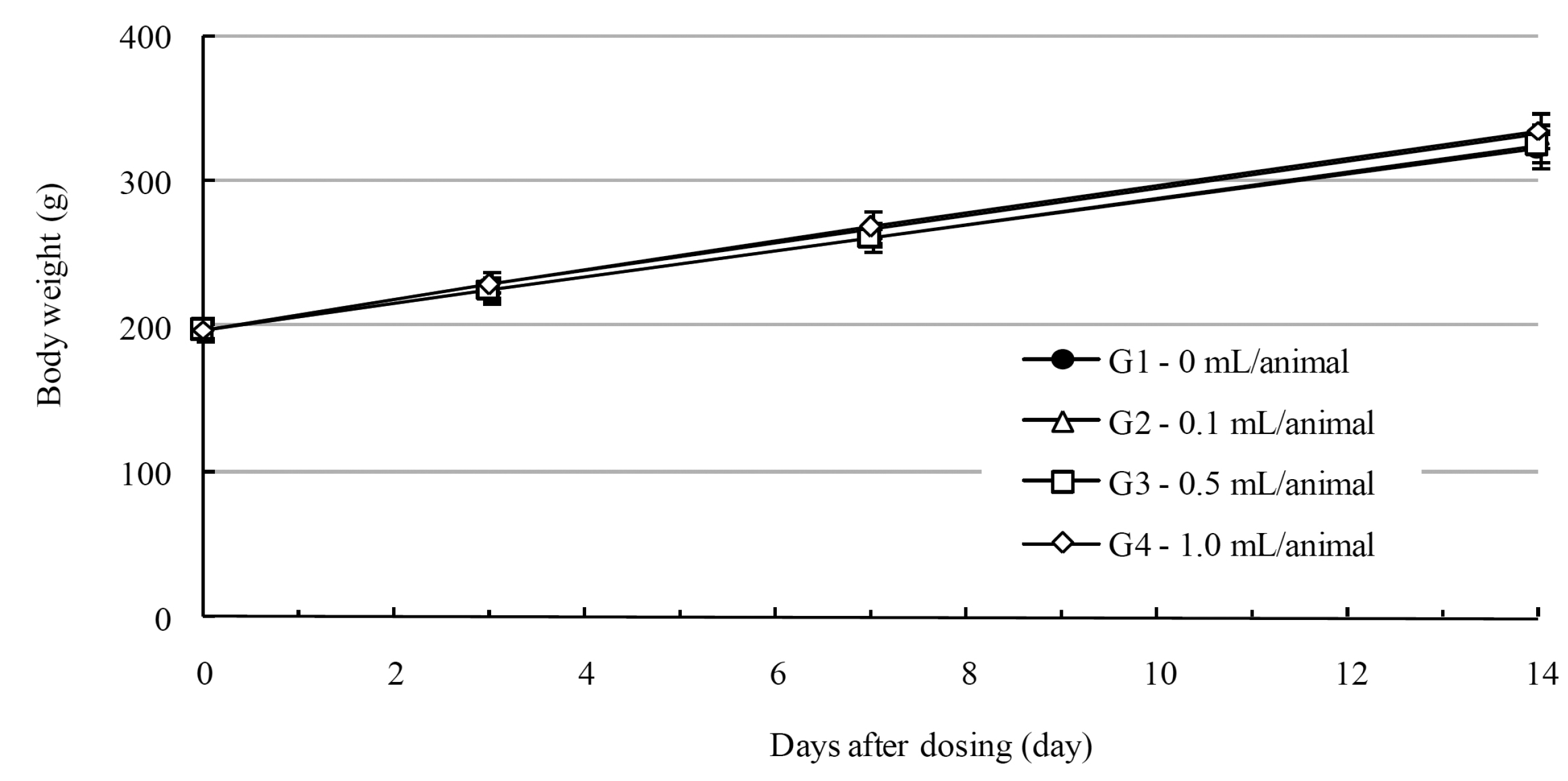
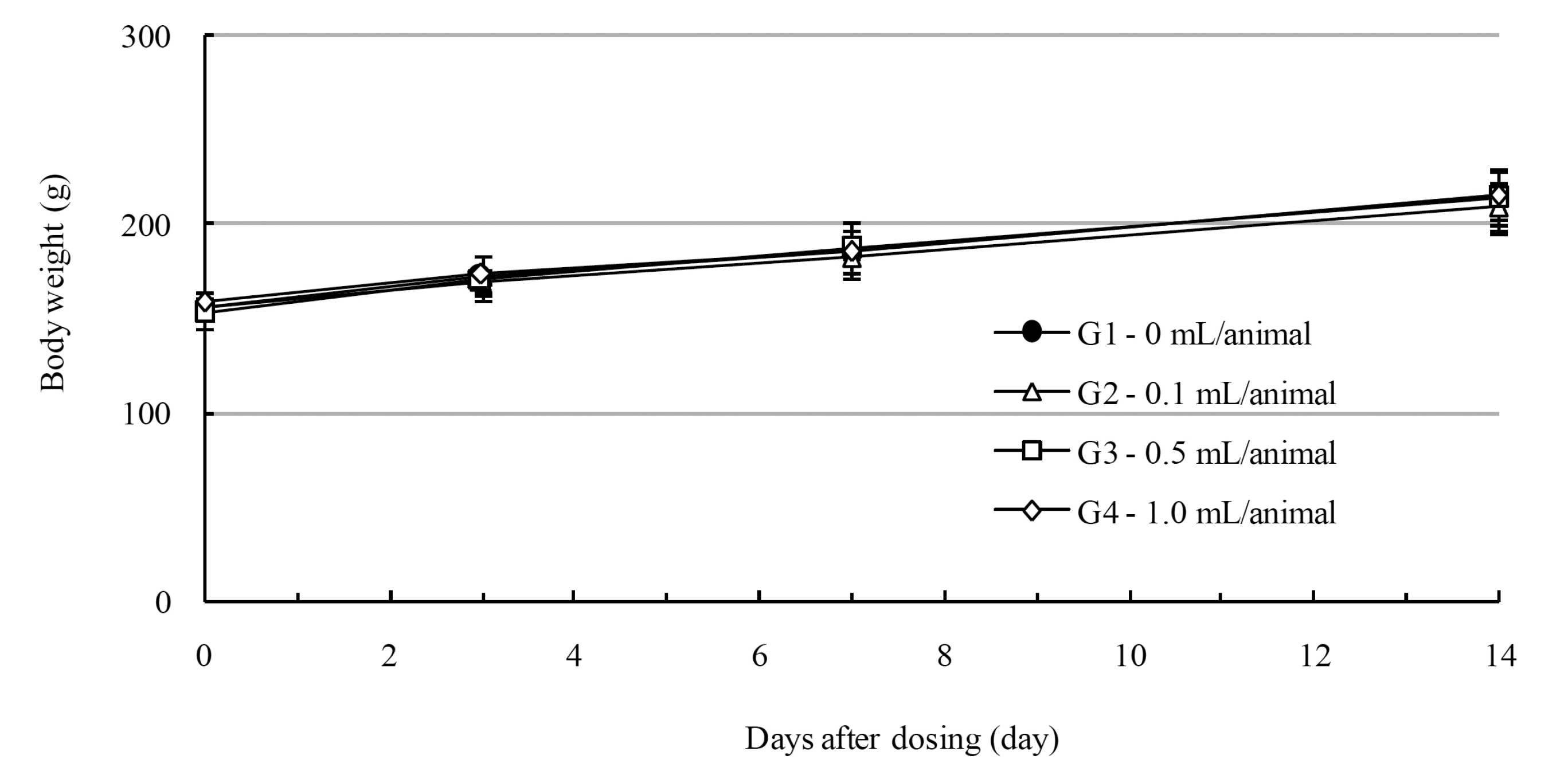
In this study, no deaths occurred in experimental rats of either sex based on this result, the LD50 of GP was assumed to be over 1.0 mL/animal. In addition, no meaningful changes in the body weights or abnormalities in the general conditions of the rats were noticed (Tables 3,4, Figs. 2,3) Furthermore, no meaningful changes in the results of the hematological tests and the biochemical tests were found (Tables 5,6) The necropsy and histopathological findings showed no abnormalities (Tables 7,8, Figs. 4-7).
Radix ginseng (Panax ginseng) has been traditionally used as an adaptogen that acts on the adrenal cortex and stimulates or relaxes the nervous system to restore emotional and physical balance and to improve well-being in patients suffering from degenerative disease and old age. Its components mostly are triterpenoid saponins, panax acid, glycosides, sterols and essential oil. Trials indicate hypoglycemic, cardiovascular [12], antiviral [13], and psychomotor enhancement [14], as well as blood pressure normalization and asthma control, properties. It appears to have antioxidant and anti-carcinogenic effects [15, 16]. In addition, we found that the enzymatic processing of ginseng saponin could increase the content of active constituents and enhance its anti-cancer activity, presumably because of the production of minor saponins, such as Rh1, Rg3, Compound K, and PPT constituents in ginseng saponin [10, 11].
Although ginseng (Panax ginseng) has often been used clinics for a long time, the safety of ginseng pharmacopuncture still needs to be tested, especially that of enzyme enforced ginseng. GP was made, and toxicity tests were performed using 0.1-, 0.5-, 1.0-mL doses of GP; the same dose of normal saline was administered to the animals in the control group. In four groups of rats in this experiment, no deaths or abnormalities on the hematological and biochemical tests were found, as was the case for the results of the necropsies and the histopathological tests. In this study, the LD50 of GP in rats was above 1.0 mL/animal, which indicates that this dose is safe.
The administering of water-soluble ginseng pharmacopuncture via a venous route in SD rats did not cause any changes in the weights, in the hematological and biochemical test and the necropsy results, or in the number of mortalities. These results indicate that venous administration of water soluble ginseng pharmacopuncture is the safe modality of treatment.
[Table. 1] Clinical characteristics of the subjects
Clinical characteristics of the subjects
[Table. 2] Summary of mortality
Summary of mortality
[Table. 3] Summary of clinical signs
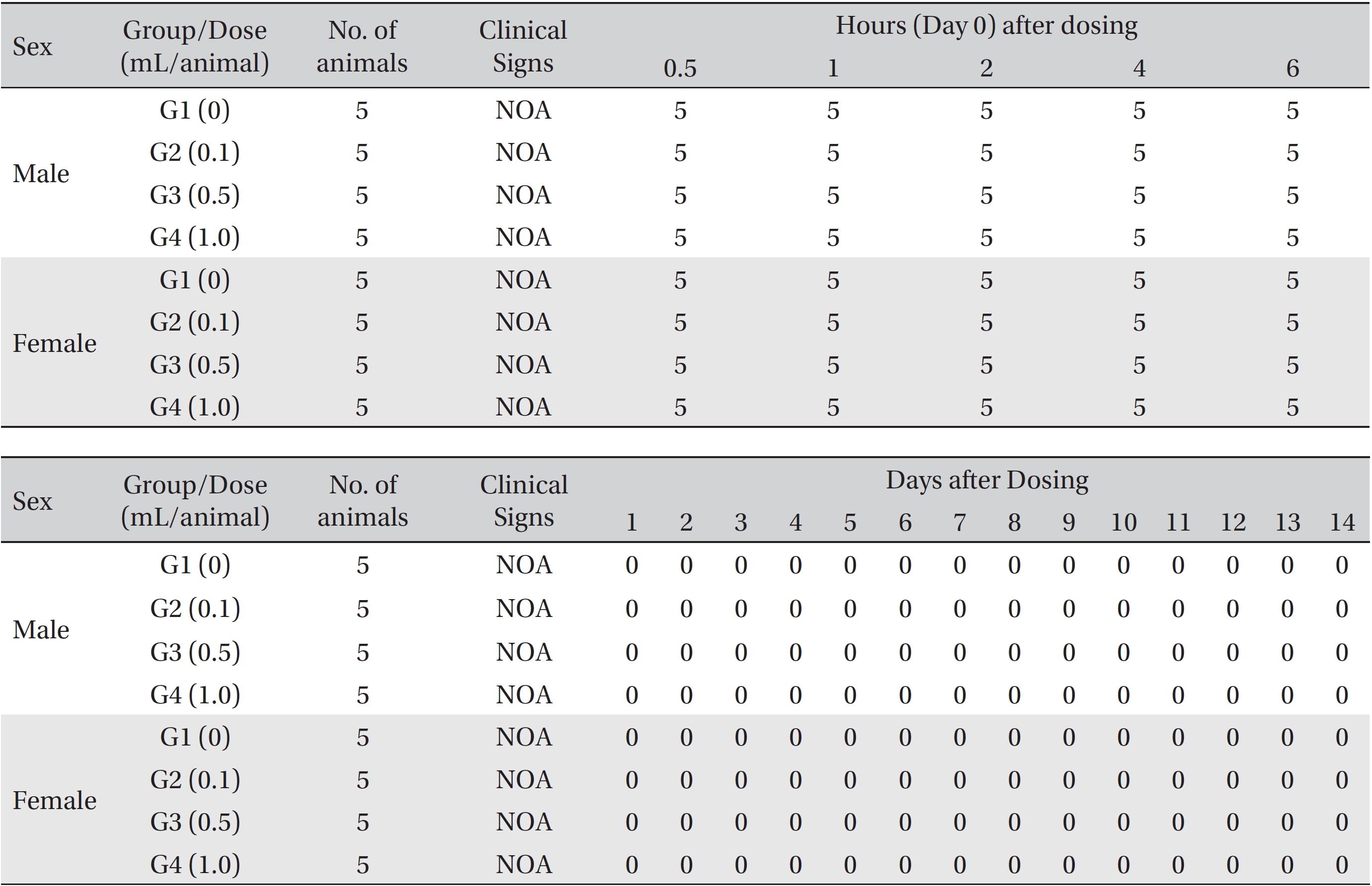
Summary of clinical signs
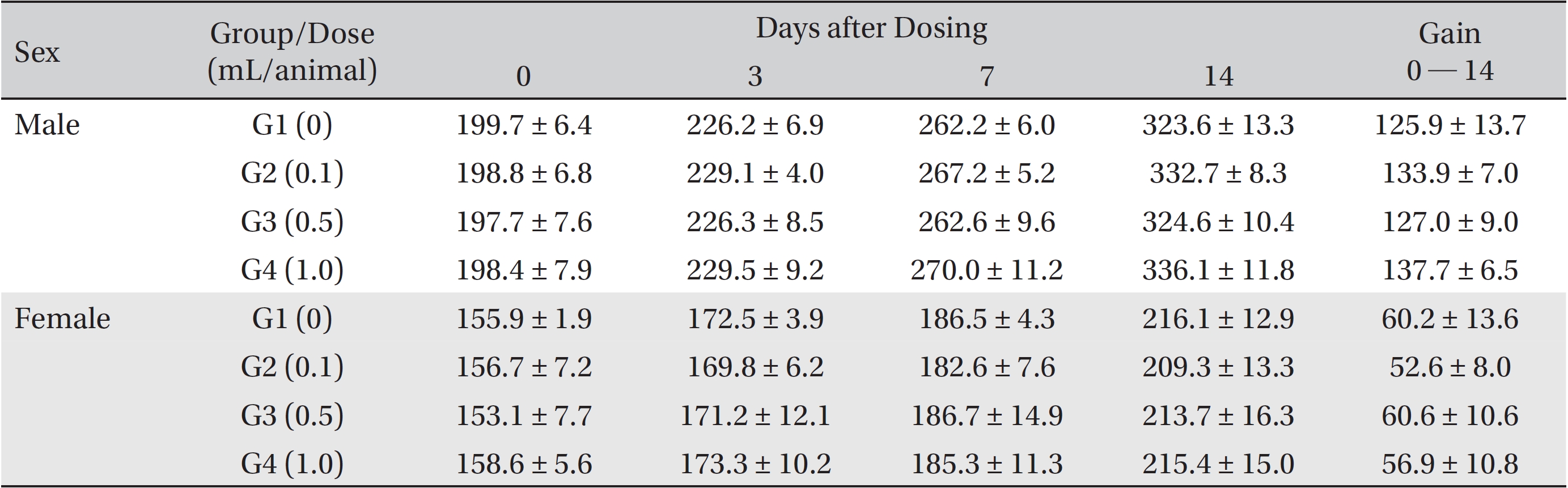
Mean body weights
[Table. 5] Mean hematology parameters in male, female Sprague-Dawley rats
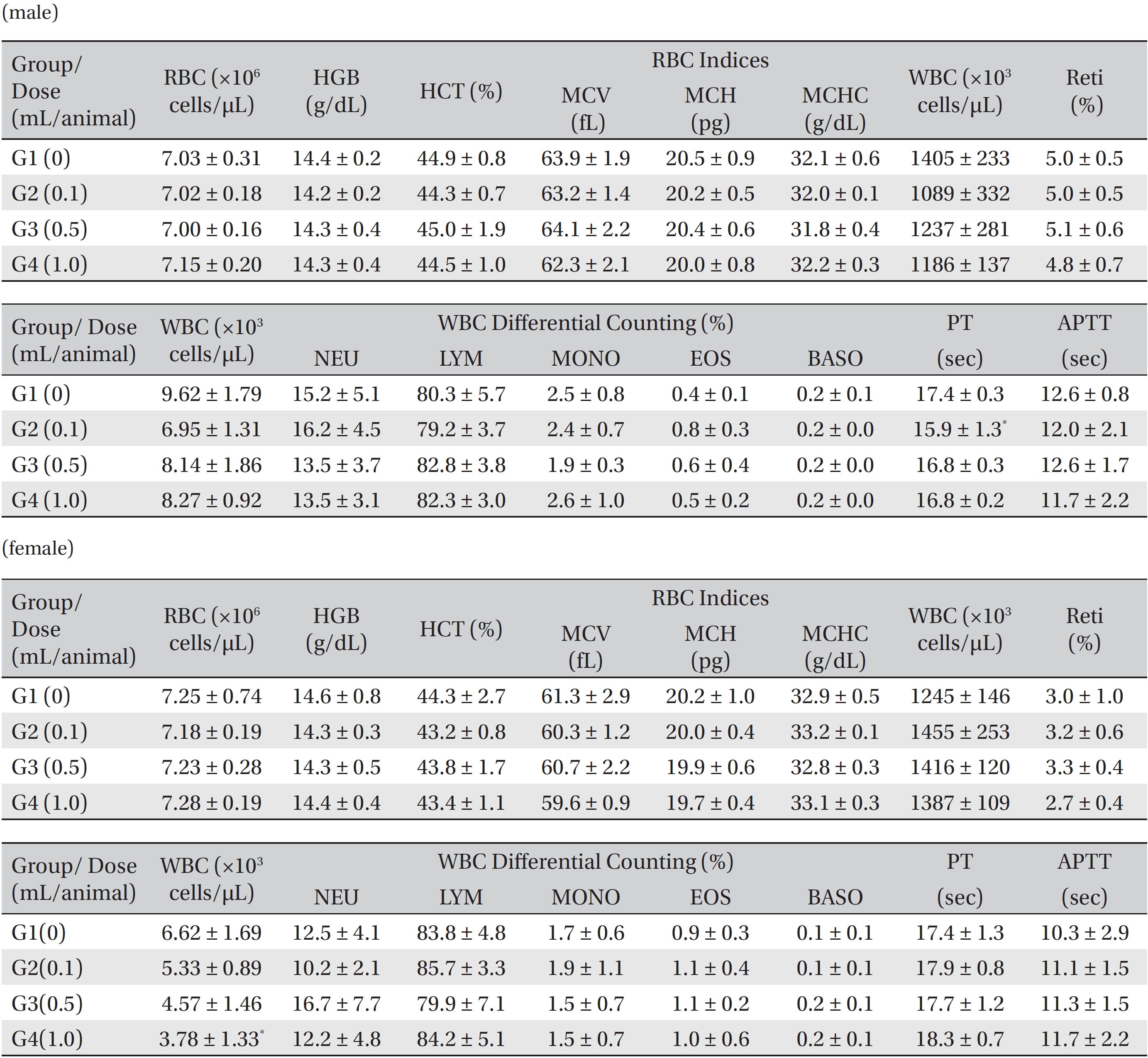
Mean hematology parameters in male, female Sprague-Dawley rats
[Table. 6] Mean clinical chemistry in male, female Sprague-Dawley rats
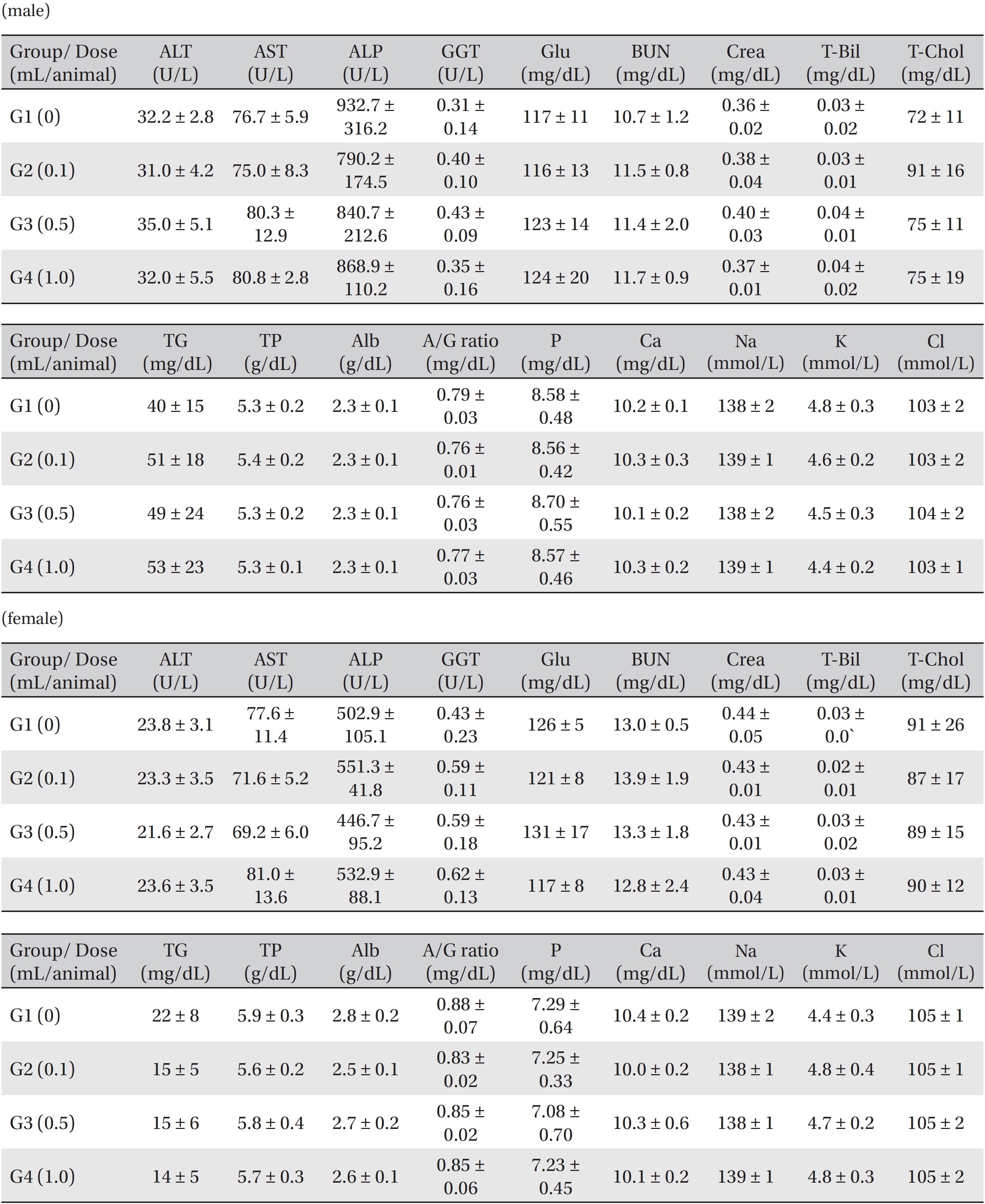
Mean clinical chemistry in male, female Sprague-Dawley rats
[Table. 7] Summary of necropsy findings
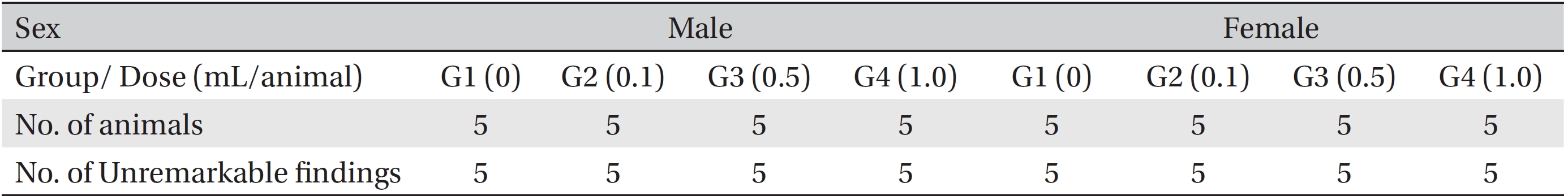
Summary of necropsy findings
[Table. 8] Summary of histopathological findings
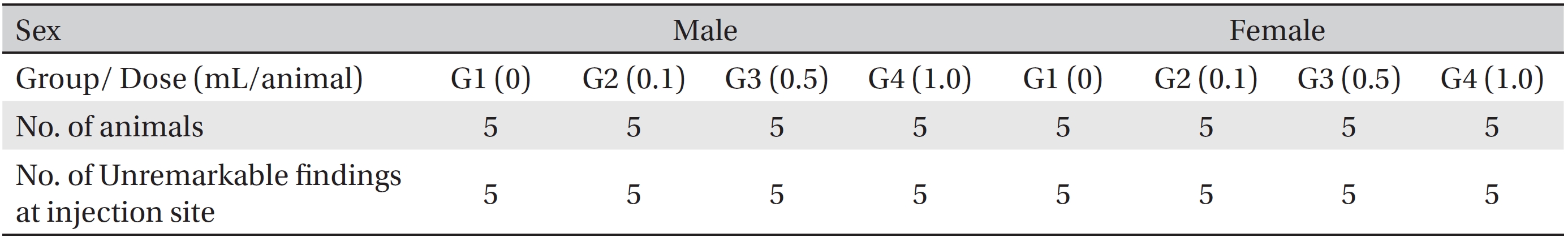
Summary of histopathological findings