Pharmacopuncture is a new type of acupuncture treatment combining acupuncture based on the meridian theory and herbal medicine based on Qi and flavor theory [1]. Pharmacopuncture is categorized as meridian field pharmacopuncture (MFP), eight principle pharmacopuncture (EPP), animal based pharmacopuncture (ABP) and mountain ginseng pharmacopuncture (MGP). Pharmacopuncture is prepared by using various methods, such as alcohol immersion, distillation, low temperature extraction, pressing, and dilution [1].
Unlike EPP and MGP, which are made by using the distillation method, pharmacopuncture for Blood and Qi recuperation (BQRP) is made by using the low temperature extraction method. The low temperature extraction method is a novel method for producing pharmacopuncture: It separates the medicinal herb extract after decocting the herb compounds. This is followed by decompression and low temperature distillation. Therefore, BQRP can maximize the inherent effect of the medicinal herbs that were processed [1, 2]. A kind of BQRP is Chukyu (spine-healing) pharmacopuncture [3], Samgihwalryeok pharmacopuncture (SGHRP) [4], Biyeon pharmacopuncture, Cheonghyeol pharmacopuncture, Eunbisan pharmacopuncture, and so on [2].
Daebohwalryeok pharmacopuncture (DHRP) is a kind of BQRP that boosts energy, and it is used for general weakness and fatigue [2]. The indications of DHRP are similar to those of MGP and SGHRP [4-6]. SGHRP has been mainly applied in clinics by using intramuscular or subcutaneous methods. DHRP has a composition similar to that of SGHRP. DHRP, with the same dosage methods and development purposes of MGP, was developed for intravenous administration to increase the effect of SGHRP. Even though toxicity is critical for intravenous injections, studies on the intravenous injection of DHRP have no yet proven its safety whereas studies on the intravenous single-dose toxicity of MGP and on the intramuscular single-dose toxicity of SGHRP have reported those pharmacopunctures to be safe under those injection conditions [4, 6]. Thus, intravenous injection toxicity tests of DHRP are necessary prior to its application. The aims of the study were to test the single- dose intravenous toxicity of DHRP in Sprague-Dawley (SD) rats and to the estimate crude lethal dose.
For the preparation of DHRP, 400 g of DHRP were extracted with 90% ethyl alcohol (EtOH) for 72 hours. The DHRP extract was obtained after the separation of extracting EtOH, which was followed by a 40°C heat treatment to evaporate the volatile EtOH. Medicinal plant powder was mixed with water for injection (WFI) at an appropriate ratio in a low temperature low pressure extractor (Fine FA, Korea). After the extravasation, the pharmacopuncture was extracted at 0.1 Torr. The extract was balanced at a pH of 7.25 ─ 7.35 and a salinity of 0.9%. The extract was passed through a N2 Gas filter (0.1 ㎛, Sartorius), was transferred to the filling tank/reservoir, and was fired in the vial for high pressure sterilization.
SD rats (Orientbio Inc., Korea) were used in this study as they are widely used for toxicity tests [3-4, 6]. Basic visual examinations were done for incoming animals, which were followed by weight measurements with an electronic scale (CP3202S, Sartorius, Germany). The general conditions were observed daily during the 7 day stabilization/ conditioning period. After the general conditions had been observed, the animals were moved to an animal room/chamber for quarantine. The body weights, general basic conditions, and weight changes were measured on the last day of stabilization to confirm/ensure the health statuses of the SD rats. At the time of intravenous injection, the weight range of the 6 week old male rats was 178.4 ─ 195.8 g (n = 15) and that of the 6 week old female rats was 148.5 ─ 169.5 g (n = 15). Breeding conditions were as follows: a temperature of 19.0 ─ 23.2°C, a relative humidity of 33.0% ─ 59.5%, a ventilation rate of 10 ─ 15 times/hour, an illumination time of 12 hours/day (7:00 am ─ 7:00 pm), and an intensity of illumination of 150 ─ 300 Lux.
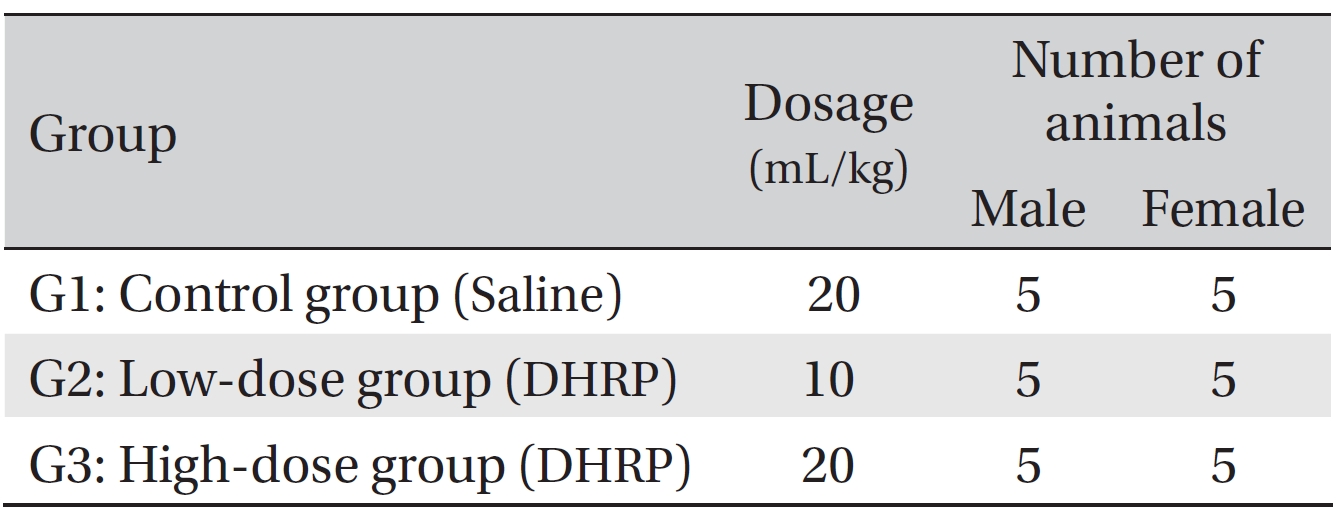
Telkad Certified Irradiated Global 18% Protein Rodent Diet 2918C (Harlan Laboratories, Inc., U.S.A.) was used to feed the animal Ad-libitum. This study was approved by the Institutional Animal Care and Use Committee of Biotoxtech Co. (Approval no: 110156). The tests were conducted according to the Good Laboratory Practice (GLP) regulation and the toxicity test guidelines of the Korea Ministry of Food and Drug Safety (MFDS). On the last day of stabilization (the grouping day), all animals whose weights were equal to the mean weight were randomly grouped into 3 groups, with 5 male rats and 5 female rats for each group. The groupings were as follows (Table 1).
DHRP were injected intravenously according to the schedule used in clinical settings. Dosages for the control and the high dose groups were 20 mL/kg each, and that for the low dose group was 10 mL/kg. For all groups, half of each dose was injected at a 2 hours interval. The initial dose for each animal was calculated based on the weight at the time of the injection. A 26G needle (3 mL) was used for all animals, and the injection speed was approximately 2 mL/minutes. The expected clinical dosage for DHRP was approximately 20 mL/human/day, which is approximately 0.33 mL/kg for an adult of 60 kg. In a pilot test (Biotoxtech Study no.: B10928P) no deaths were observed for single-dose intravenous injections of 20 mL/kg and 10 mL/kg in male and female rats. As a result, dosages for this study were set at 20 mL/kg, maximum available dose, for the high dose group, which is approximately 60 times the expected clinical dosage, and 10 mL/kg for low dose group. Normal saline (Choongwae Pharma Corp., Korea) was injected into the rats in the control group.
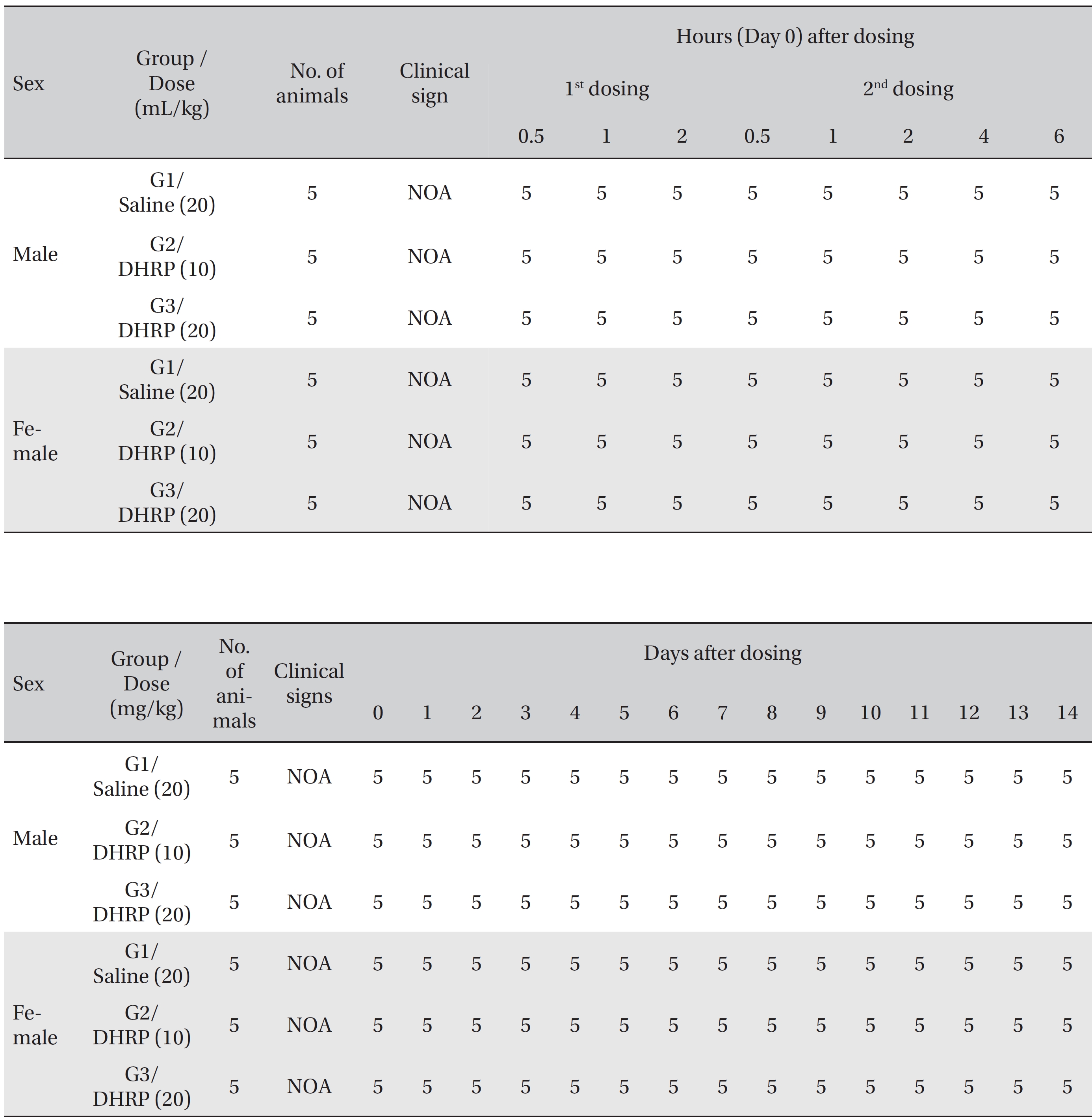
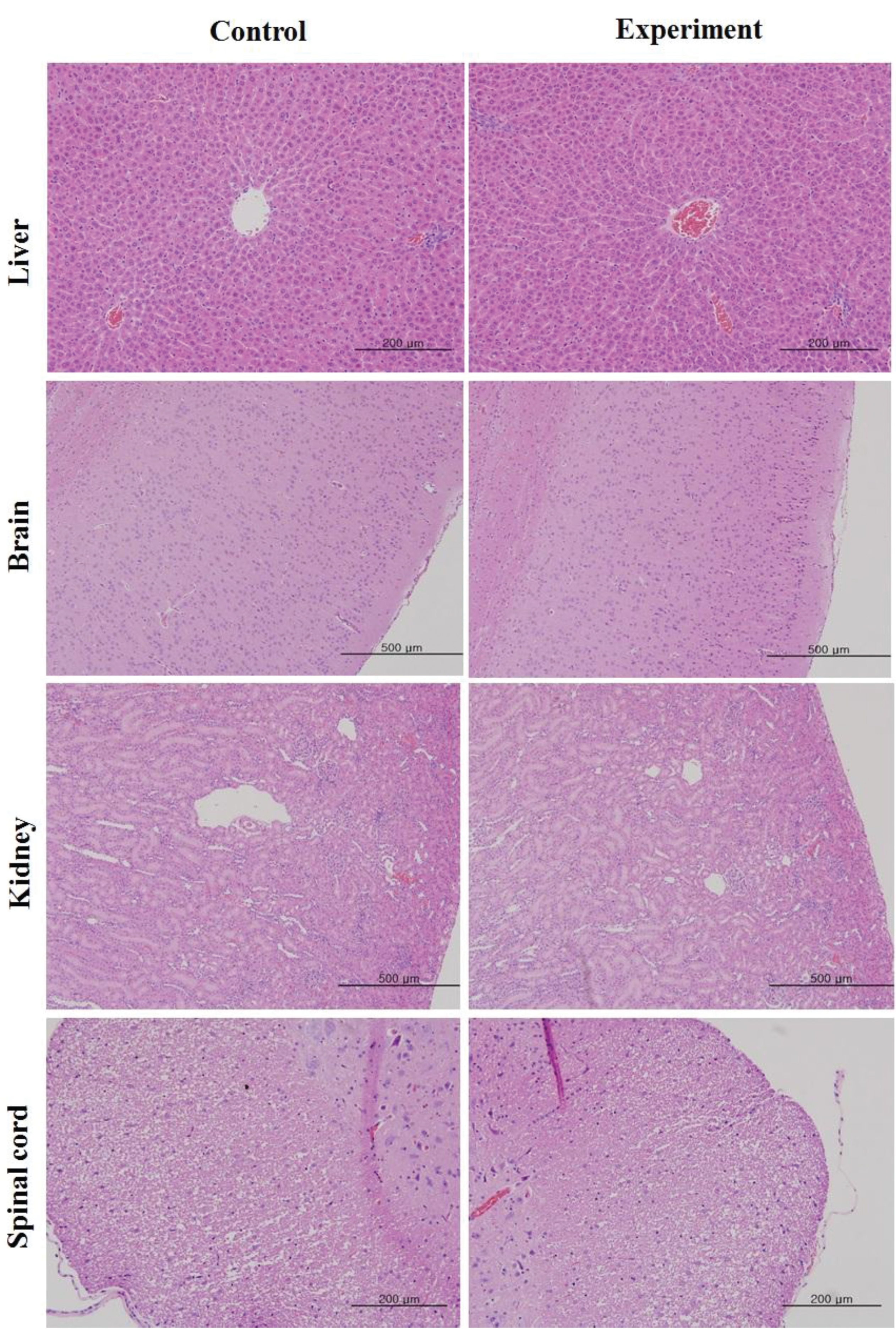
At the day of injection (day 0), general conditions (types of toxicity, onset time, recovery time) and deaths were observed 30 minutes, 1 hour, and 2 hours after the first injection, and at 30 minutes, 1, 2, 4, and 6 hours after the second injection. General conditions were noted daily for 14 day after injection. Body weights were measured on the day of the injection (before injection) and on the third, seventh, and fourteenth (necropsy day) days after injection. After the observation period, necropsy with CO2 gas anesthesia, followed by abdominal aorta bloodletting, was conducted for all animals. Organs and tissues, including the brain, heart, liver, spleen, kidney, lung, and spinal nerve, were extracted from sacrificed animals and fixed with neutral buffered formalin solution. After the organs and tissues had been fixed, sections were produced with dehydrated paraffin. Histotomies were dyed with hematoxylin & eosin (H&E) solution. Residual organ, tissue and fixed organ, tissue were preserved with 10% neutral buffered formalin solution. Microscopic examinations were performed for all sections made for histotomy in both the control group and the high dose group.
Data were analyzed with statistical analysis system (SAS, version 9.2, SAS Institute Inc., U.S.A). A homoscedasticity check was done with Bartlett test (significance level of 0.05). When homoscedasticity was satisfied, a one-way analysis of variance (ANOVA) was done, followed by a Dunnett's
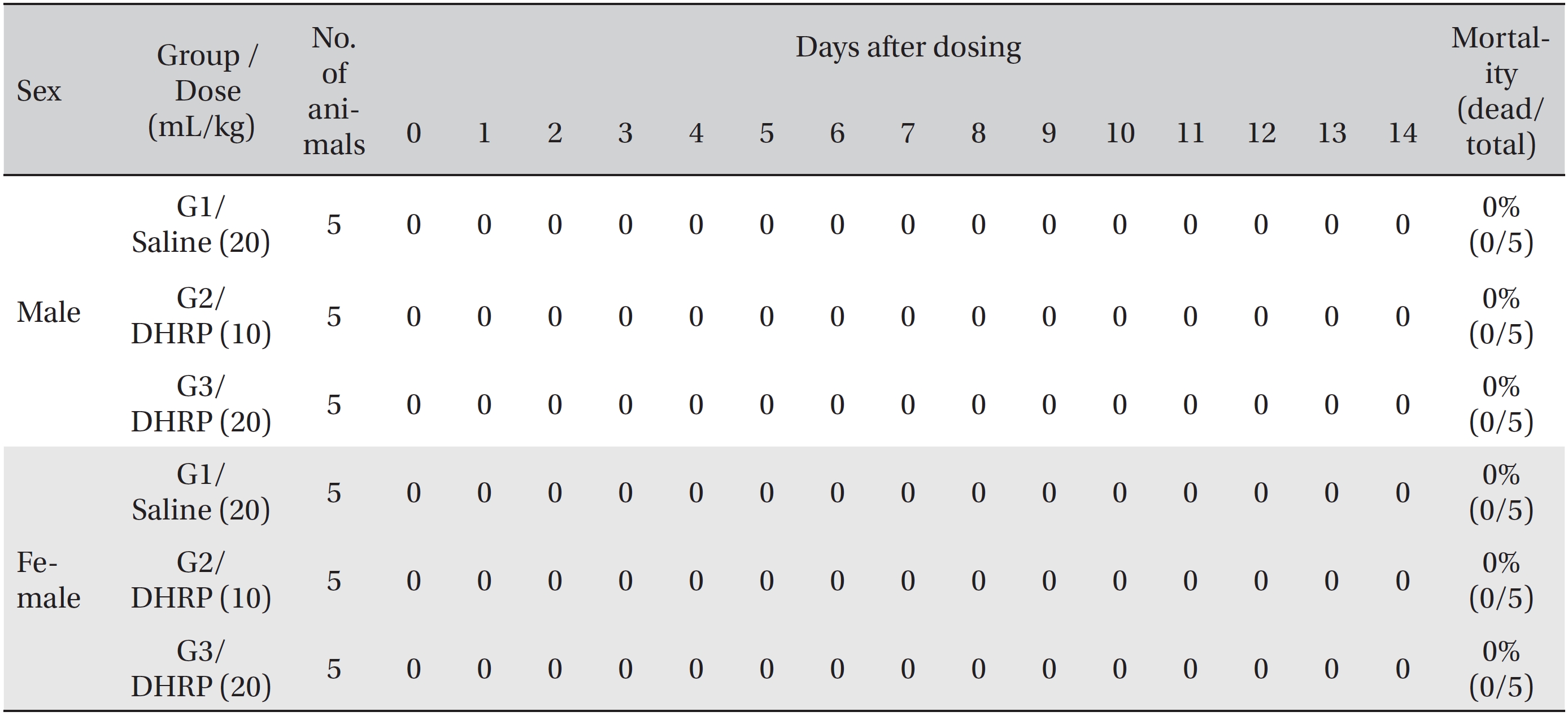
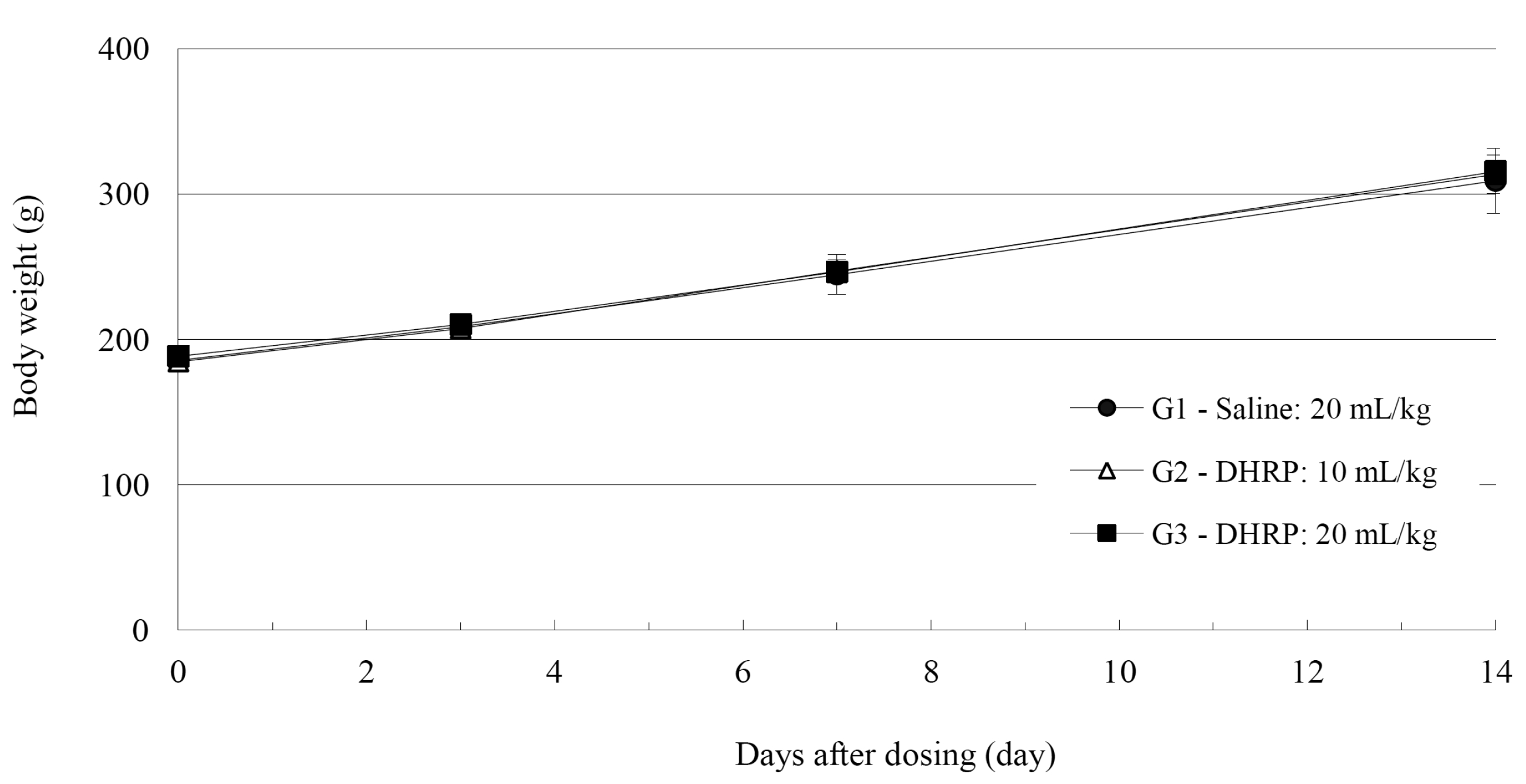
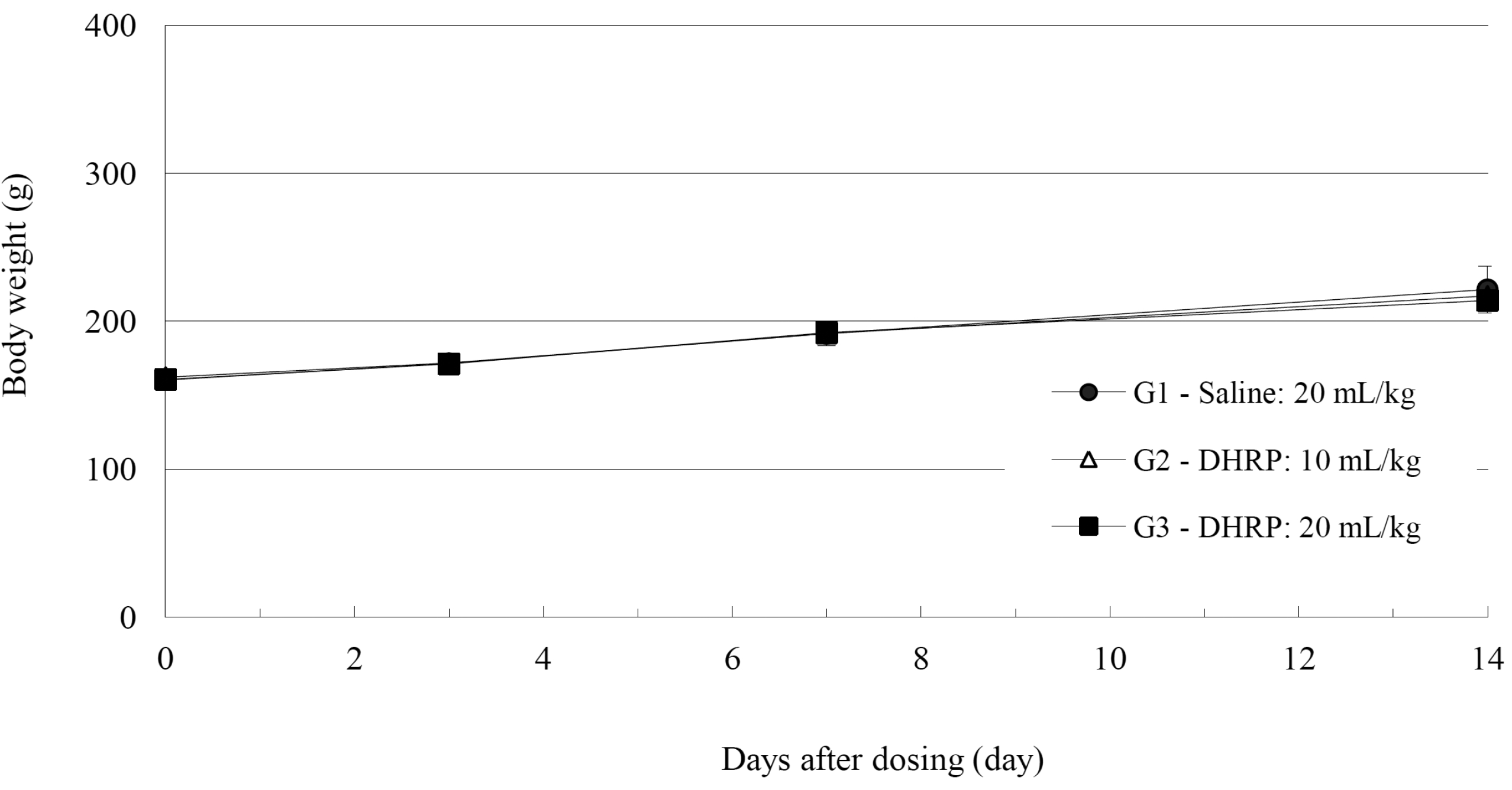
No deaths were observed for both male and female rats in the control and the test groups during the observation period (Table 2). In addition, no abnormal conditions were observed for both male and female rats in the control and the test groups during the observation period (Table 3). Moreover, no statistically significant weight changes were observed for both male and female SD rats in the test groups, compared with the SD rats in the control group, during the observation period (Figs. 1,2).
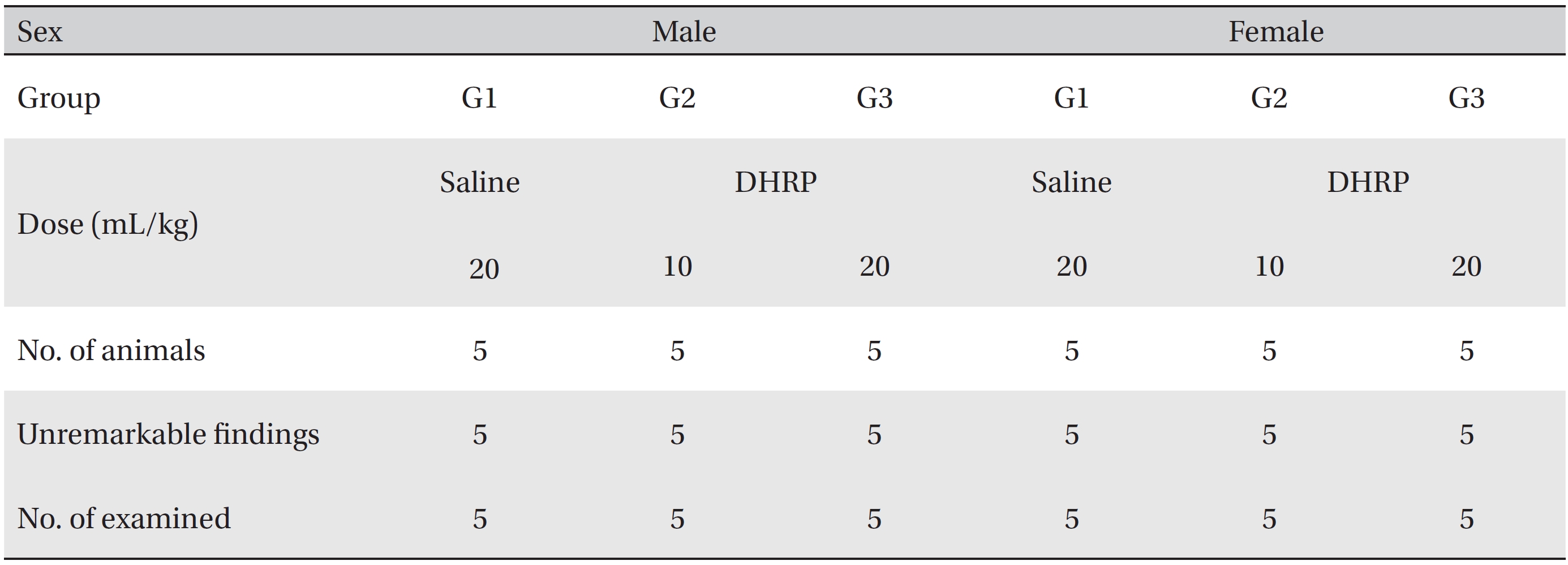
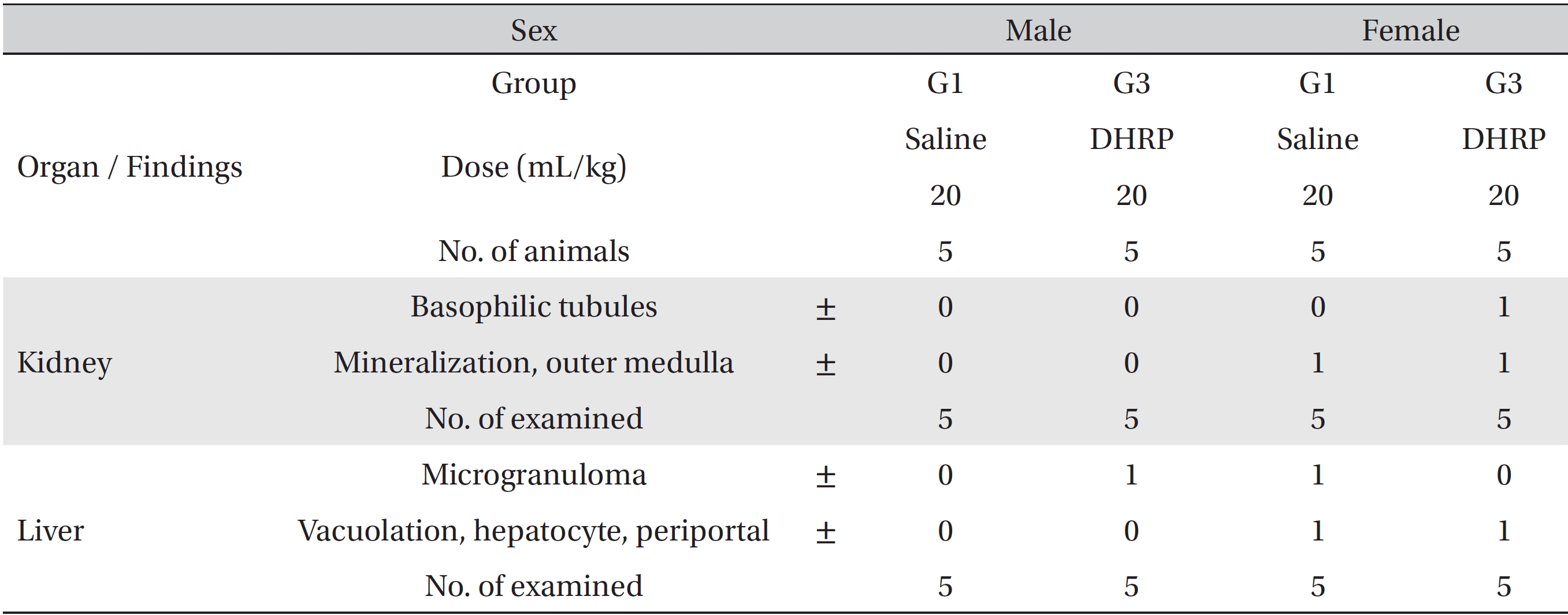
As a result of necropsy, no macroscopic abnormalities were observed in the control and the test groups during the observation period (Table 4). No DHRP related effects were observed in the 20 mL/kg DHRP group for both male and female rats. Other findings that were observed in the kidneys and the livers in the control and the high dose groups, which were spontaneous and adventitious ones, were lesions commonly observed in age relevant SD rats and had no toxicological significance (Fig. 3, Table 5).
The composition of DHRP is equal to that of SGHRP, which consists of
CC is the hairy, young horn of a male deer or stag,
AGR is the root of
OR is the root of
SF is the dried fruit of
On the basis of the effect of the above herbs, DHRP can greatly tonify both the Qi and the blood system of the body. DHRP has an effect similar to that of SGHRP because DHRP has the same composition as the SGHRP herbs that tonify Qi, blood, fluid and humor. Thereby, we postulate that not only does DHRP produce healthy circulation that results in enhanced yang Qi, but also it can applied to treat diverse consumptive or intractable diseases due to Qi deficiency, blood deficiency, and Qi and blood deficiency, such as lassitude, gastric atony, chronic fatigue, stroke and cancer.
As MGP was developed for intravenous injection to enhance the effect of tonifying Qi and blood [6], DHRP was developed for intravenous administration to improve the effect of SGHRP [4]. Not only has MGP been widely used intravenously in clinics in Korea, but also the safety of MGP has been proven by using intravenous single-dose toxicity test [6].
On the basis of our single-dose toxicity study, we conclude that intravenous injection of DHRP can be safe because all SD rats showed tolerance to doses over 20 mL/ kg. We secured the basic safety evidence that DHRP can be applied intravenously to patients with deficiency. However, further safety studies, such as 4-week recovery tests, 13- week, repeated intravenous dose toxicity tests and so on, and further efficacy studies will be needed in order to provide more conclusive results. In addition, we think DHRP can be injected into subcutaneous tissue or muscle to treat diseases, just as SGHRP can [4], because DHRP consists of SGHRP and has the same applications in clinics. Thus, safety and efficacy studies for different dose methods will be needed.
Under the conditions of this study, single-dose intravenous injection of DHRP is safe because lethal doses were estimated to be over 20 mL/kg for both male and female rats.
[Table. 1] Grouping of animals
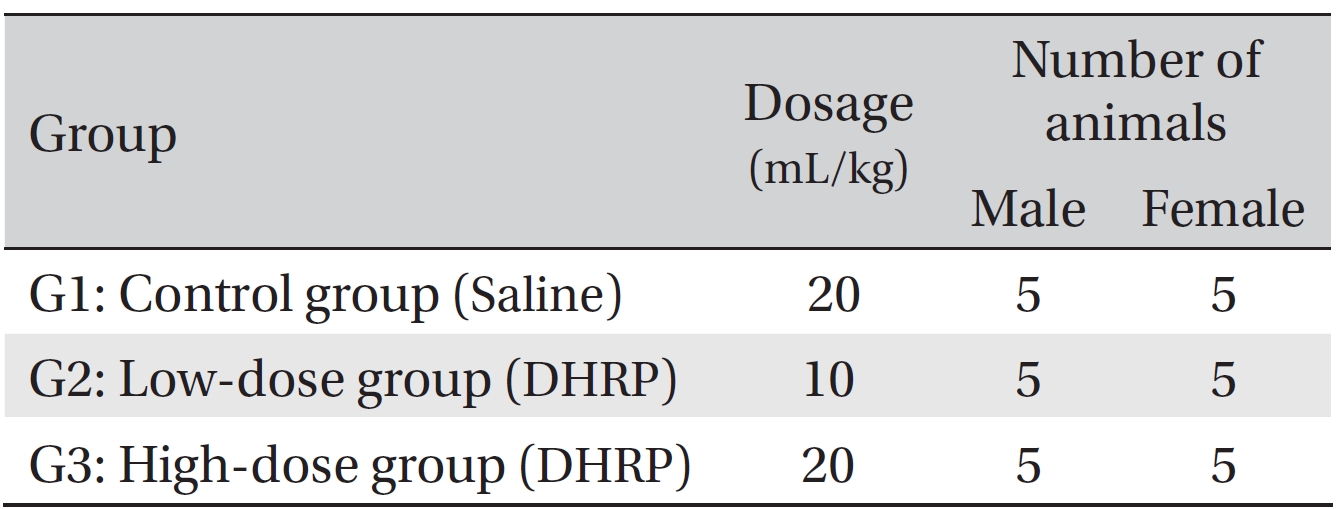
Grouping of animals
[Table. 2] Summary of mortalities
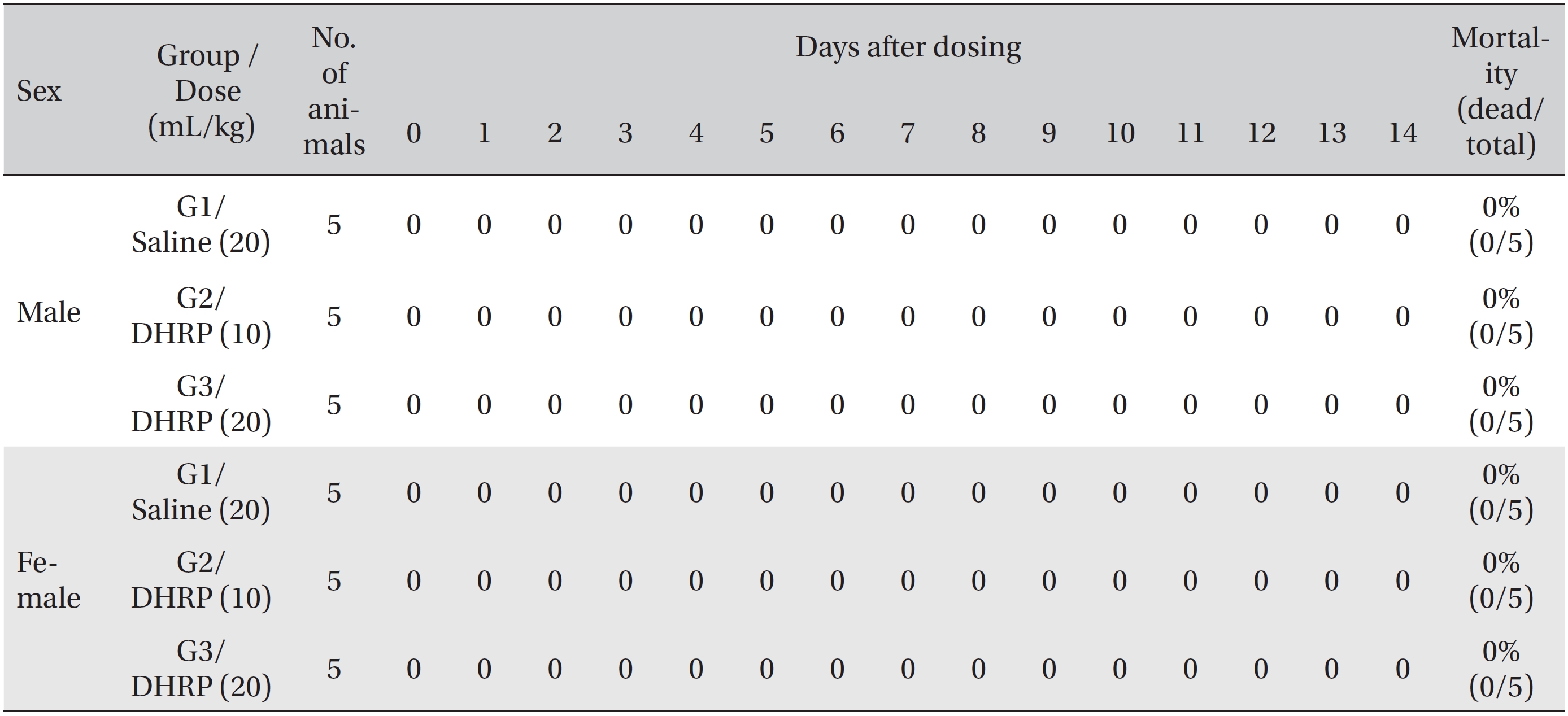
Summary of mortalities
[Table. 3] Summary of clinical signs
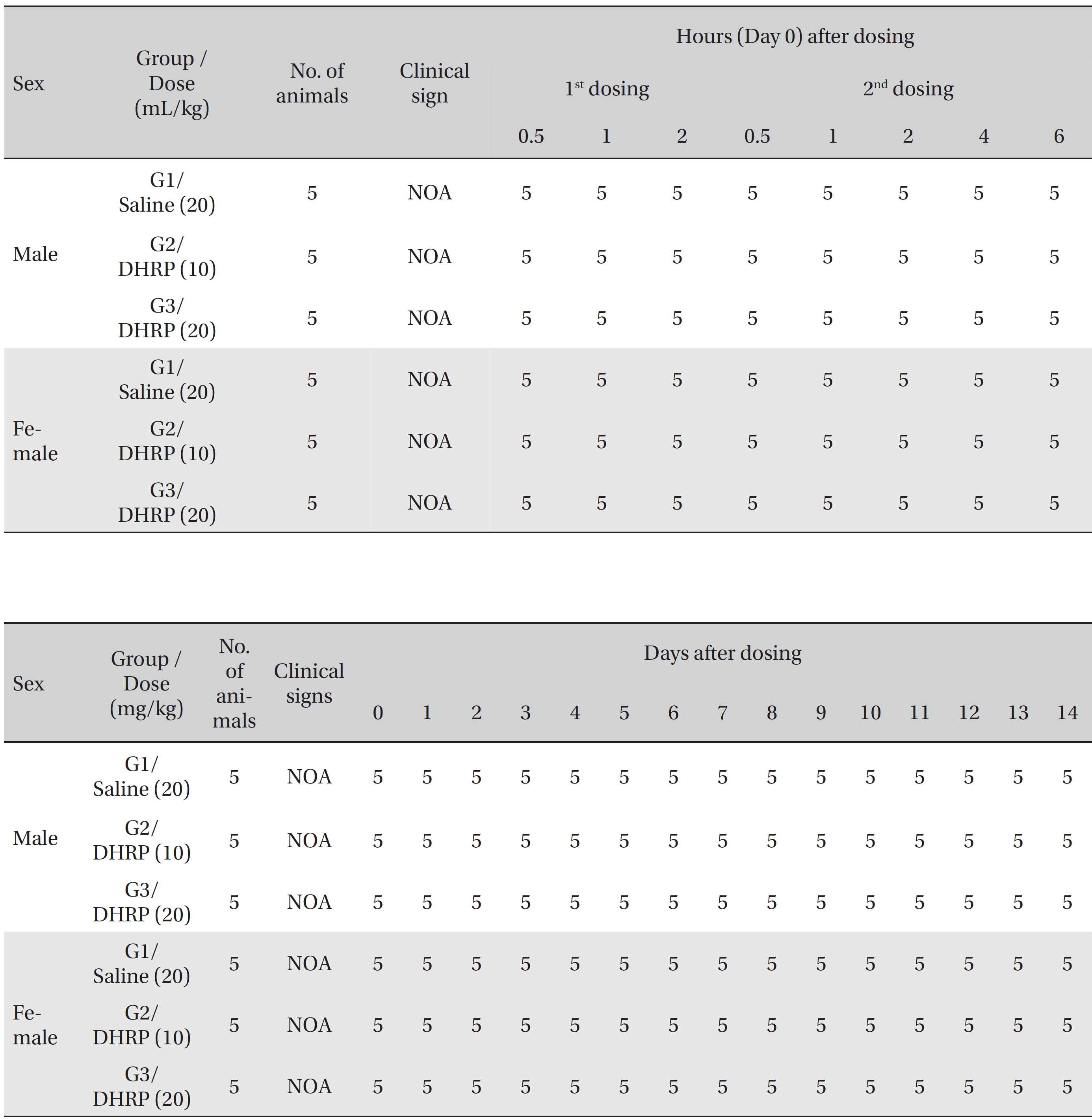
Summary of clinical signs
[Table. 4] Summary of necropsy findings
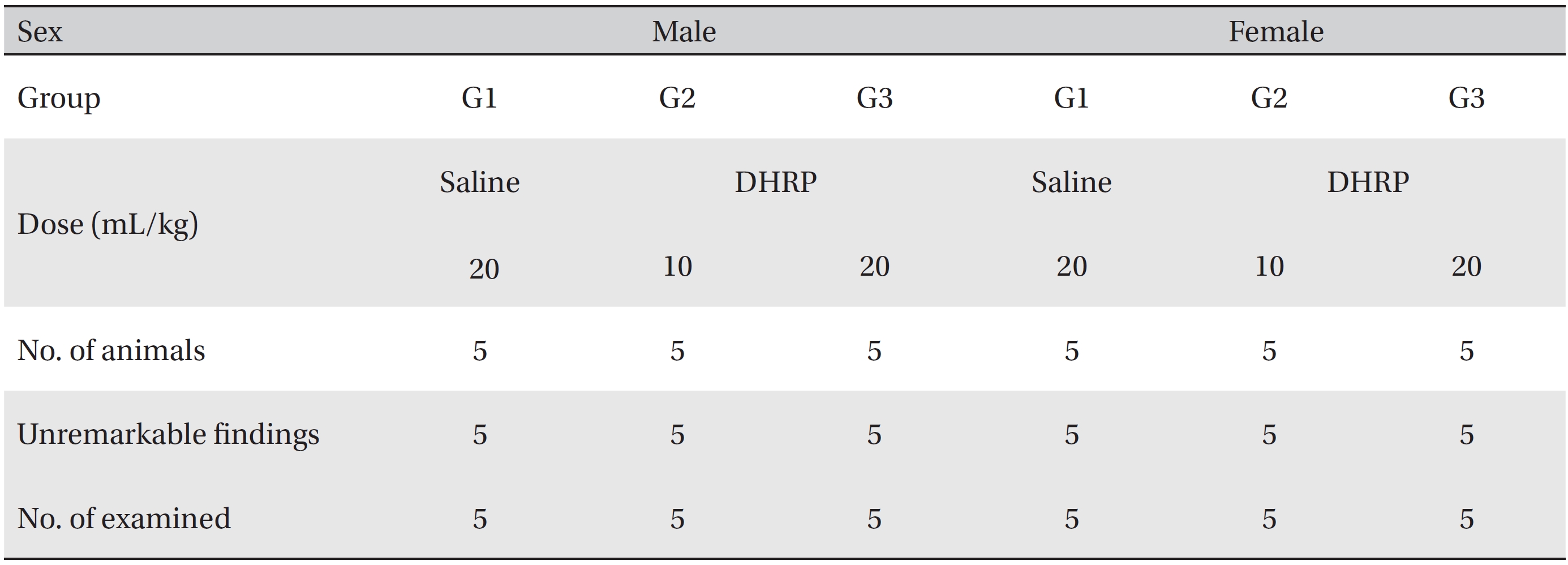
Summary of necropsy findings
[Table. 5] Summary of histopathological findings
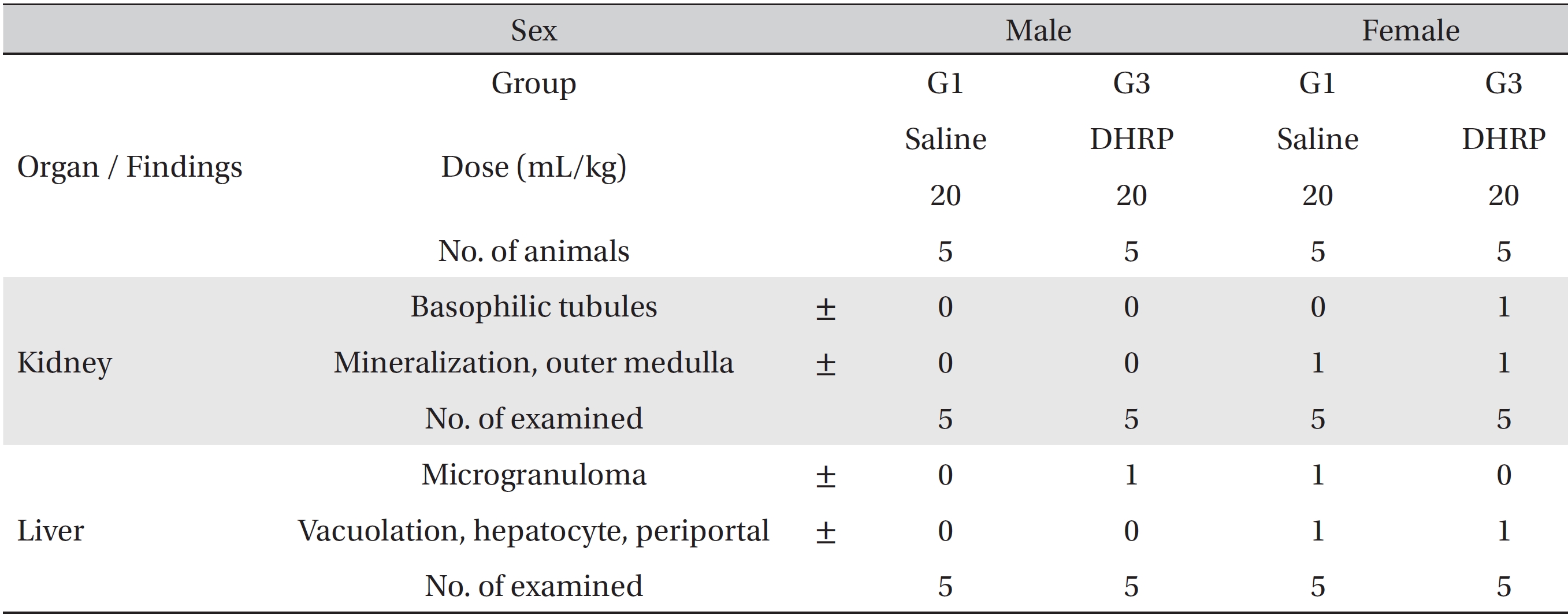
Summary of histopathological findings