A vertebral compression fracture (VCF) is characterized by back pain and fracture of a vertebral body on spinal radiography. VCFs of the thoraco lumbar spine are common in the elderly. In general, appropriate analgesics should be prescribed to reduce pain and, thus, promote early mobilization. The ideal treatment approach for VCFs has not been determined. In Korea, acupuncture and herbal medication have been used to treat VCFs for many years. There is empirical evidence that acupuncture might benefit patients with a VCF. However, no randomized, controlled, clinical trials evaluating the efficacy and the safety of acupuncture for treating a VCF have been published. Therefore, we designed a randomized, controlled, pilot, clinical trial to obtain information for the design of a further full scale trial.
A five week protocol for a randomized, controlled, pilot, clinical trial is presented. Fourteen patients will be recruited and randomly allocated to two groups: a control group receiving interlaminar epidural steroid injections once a week for three weeks, and an experimental group receiving interlaminar epidural steroid injections plus acupuncture treatment (three acupuncture sessions per week for three weeks, nine sessions in total). The primary outcomes will be the pain intensity (visual analogue scale and PainVisionTM system). The secondary outcome measurements will be the answers on the short form McGill pain questionnaire and the oswestry disability index. Assessments will be made at baseline and at one, three, and five weeks. The last assessment (week five) will take place two weeks after treatment cessation. This study will provide both an indication of feasibility and a clinical foundation for a future large scale trial. The outcomes will provide additional resources for incorporating acupuncture into existing treatments, such as nonsteroidal anti-inflammatory medications, narcotics and vertebral augmentation. This article describes the protocol.
A vertebral compression fracture (VCF) is characterized by back pain and a fracture of a vertebral body on spinal radiography [1, 2]. VCFs of the thoraco lumbar spine are common in the elderly, with approximately 1.5 million VCFs annually in the general US population [3]. The most common etiology of a VCF is osteoporosis, although trauma [4], infection, and neoplasm can also lead to a VCF [5, 6]. Even though most VCFs are benign, a subset may cause significant morbidity and serious public health problems with important socio economic effects [7, 8]. In the 1990s, the estimated annual medical cost of VCFs in the U.S. was $746 million [9].
Although the medical history and a physical examination may reveal symptoms suggestive of a VCF, the diagnosis must be confirmed with a spinal imaging study [10]. Anteroposterior and lateral radiography of the lumbar or the thoracic spine are the first investigations to be performed. Radiographic evaluation of VCFs may demonstrate the classic wedge fracture, which shows a loss of anterior vertebral body height with relative preservation of posterior vertebral body height. However, in the absence of an obvious vertebral deformity, bone scans or magnetic resonance imaging (MRI) may be required to make a definitive diagnosis [11-13]. To date, the best management of acute painful vertebral fractures is unclear [12].
Once a VCF has been diagnosed, nonsurgical management with activity modification and symptomatic medication, with or without bracing, is adequate for a majority of patients [7]. Tailoring medications to the patient’s needs based on their pain level and type is important [14]. Appropriate analgesic medications should be prescribed to reduce pain and allow daily living activities. First line medications include acetaminophen, salicylates, or nonsteroidal anti-inflammatory medication. Narcotics should be reserved for patients failing to obtain adequate relief with the above regular analgesics [7]. In addition to the above outlined treatment, some patients may require further intervention, such as epidural steroid injections, to reduce pain and improve function [14, 15]. Surgical intervention is indicated in cases where patients have intractable back pain even after conservative therapy, where there is evidence of an impending or existing neurologic deficit, or where the spinal deformity is extremely severe [16, 17]. Vertebral augmentation through minimally invasive techniques, such as kyphoplasty and percutaneous vertebroplasty, are among the most popular [16, 18].
Acupuncture has become a popular alternative for the treatment of back pain, which is the major symptom of a VCF. In Korea, acupuncture and herbal medication have been used to treat VCFs for many years. Importantly, acupuncture has been shown to be effective in relieving low back pain [19, 20]. Leibing
A protocol for a randomized, controlled, pilot, clinical trial is presented. This protocol adheres to the principles of the declaration of Helsinki and has been approved by the Institutional Review Board of Daegu Catholic University Hospital (IORG0004453), where the study will take place. The trial is registered with the U.S. National Institutes of Health Clinical Trials Registry (NCT01913587). Written consent will be obtained from each participant before any treatment is given.
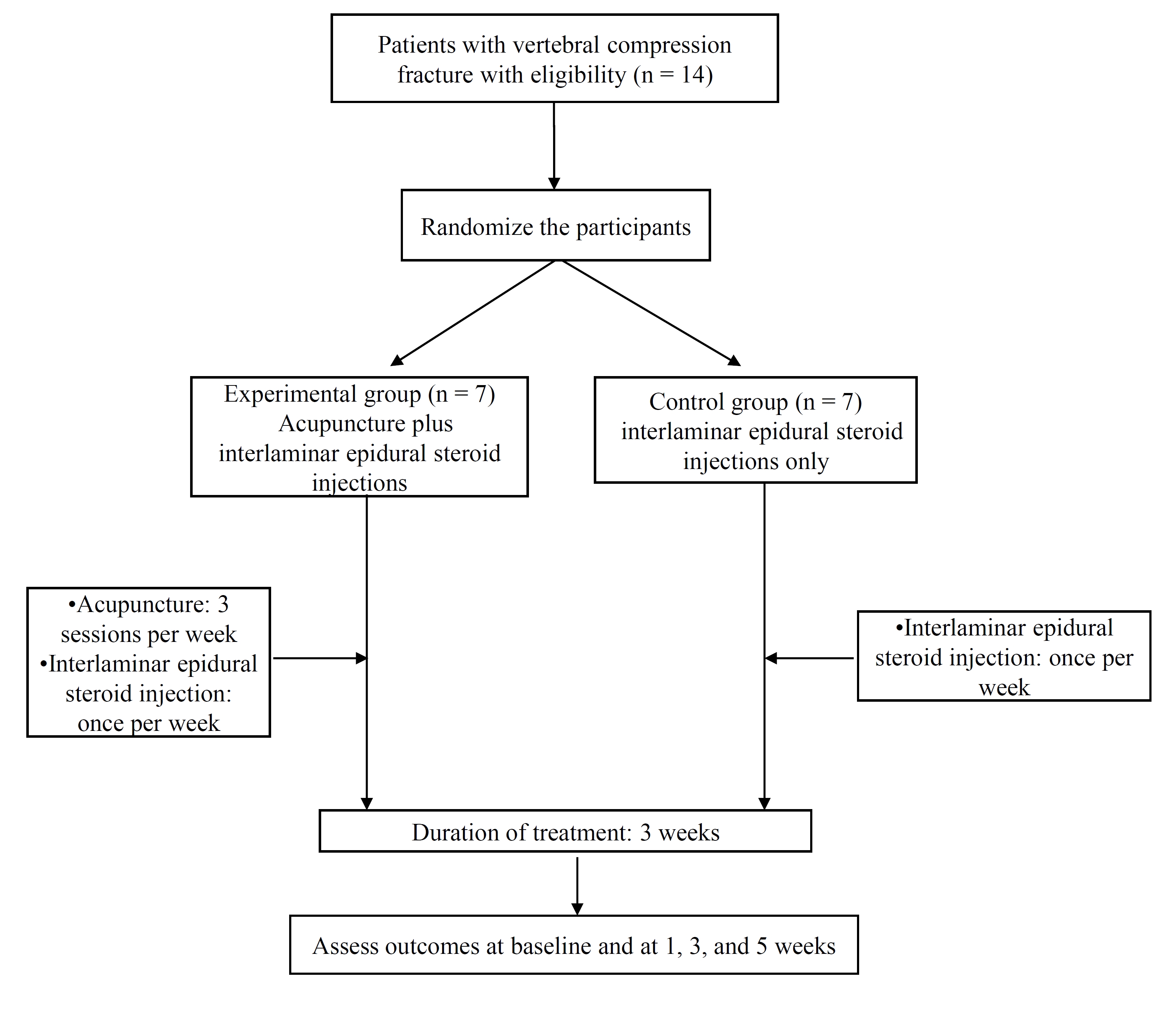
The outcome assessments and statistical analyses will be performed by professionals blinded to the assignment of patients. The trial protocol is presented in (Fig. 1). The trial will run for five weeks. Patients will be randomly allocated to two groups: a control group, in which patients will receive three interlaminar epidural steroid injections for three weeks, and an experimental group, in which patients will receive the same interlaminar epidural steroid injections as well as nine acupuncture sessions (three sessions per week for three weeks). Assessments will be made at baseline, and again at one, three, and five weeks. The last assessment (week five) will take place two weeks after treatment cessation.
Participants will be recruited through advertisements on hospital websites and on bulletin boards. If patients are interested in participation, they will be invited to visit the hospital for a screening meeting. Their eligibility will be determined by an anesthesiologist through physical and radiological examinations. If eligible, they will be guided through the informed consent process. After written consent has been obtained, a study researcher will randomly allocate the participants to one of the two groups. Treatments will be scheduled after randomization.
This study is designed as pilot study, and a 20% dropout rate is expected. A target of 14 patients with a VCF has been set. Each group will include seven patient. Inclusion criteria are as follows: a VCF caused by trauma or osteoporosis; a minimum 15% loss of vertebral height; diagnosis by X-ray, computed tomography (CT), or MRI; a VAS score of 5 or more; age over 50; recruitment at 2 weeks or more from onset; follow-up possible during the clinical trial; voluntary written informed consent. Exclusion criteria are as follows: recruitment within 2 weeks of onset; pathological fracture due to malignancy (myeloma) or osteomyelitis; major retropulsion of bony segments into the spinal canal; bone metabolic disease; significant renal or hepatic disease; hypersensitive reaction to acupuncture treatment; senile dementia, impaired cognitive function or other cerebral disease, and severe psychiatric or psychological disorders; alcohol/drug abuse; ineligibility as determined by an anesthesiologist; refusal to participate in the trial or to provide informed consent; any contraindication to corticosteroid injection (e.g., insulin dependent diabetes); inability to comprehend or express oneself in the Korean language.
Patients will be randomized using a computerized random number generator by an independent statistician blinded to patient assignment in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement. Block randomization will be performed once the participants are confirmed to be eligible, and their written informed consent has been obtained.
Patients will be randomly divided into two treatment groups: a control group (interlaminar epidural steroid injection) and an experimental group (interlaminar epidural steroid injection plus acupuncture). The interlaminar epidural steroid injections will be administered once per week for three weeks (three times in total), and the acupuncture sessions will be performed three times per week for three weeks (nine times in total). If patients take both interlaminar epidural injection and acupuncture, acupuncture will be taken 30 minutes after finishing the interlaminar epidural injection.
2.5.1. Interlaminar epidural steroid injection
The lumbar interlaminar epidural injections will be administered by an anesthesiologist under fluoroscopy in a sterile procedure room in an outpatient pain management department. The lumbar interlaminar epidural space will be identified by using the loss of resistance technique and by using fluoroscopic visualization with confirmation by using iohexol contrast medium. The epidural needle will be entered at the level of the vertebral compression fracture or one space below the level of the compression fracture. After confirmation of needle placement, 10 mL of a solution containing 2 mL of 0.5% preservative free bupivacaine hydrochloride, 10 mg of non particulate dexamethasone, and normal saline will be administered by injection.
2.5.2. Acupuncture treatment
The following acupoints will be used: two back transporting acupoints (located on the superior and the inferior sides of the fractured vertebral body), BL40, BL60, GB34, and ST36 (bilateral). In total, 12 acupoints will be used. Sterilized disposable acupuncture needles (0.25 mm × 40 mm in size; DongBang Acupuncture Inc., Korea) will be manually inserted into the acupoints. After needle insertion, the De Qi sensation will be induced by using manual stimulation, and four back transporting acupoints will be stimulated by using an electro acupuncture device (ES-160; Ito Co. Ltd., Japan). The needles will be inserted for 20 ± 5 minutes and then removed.
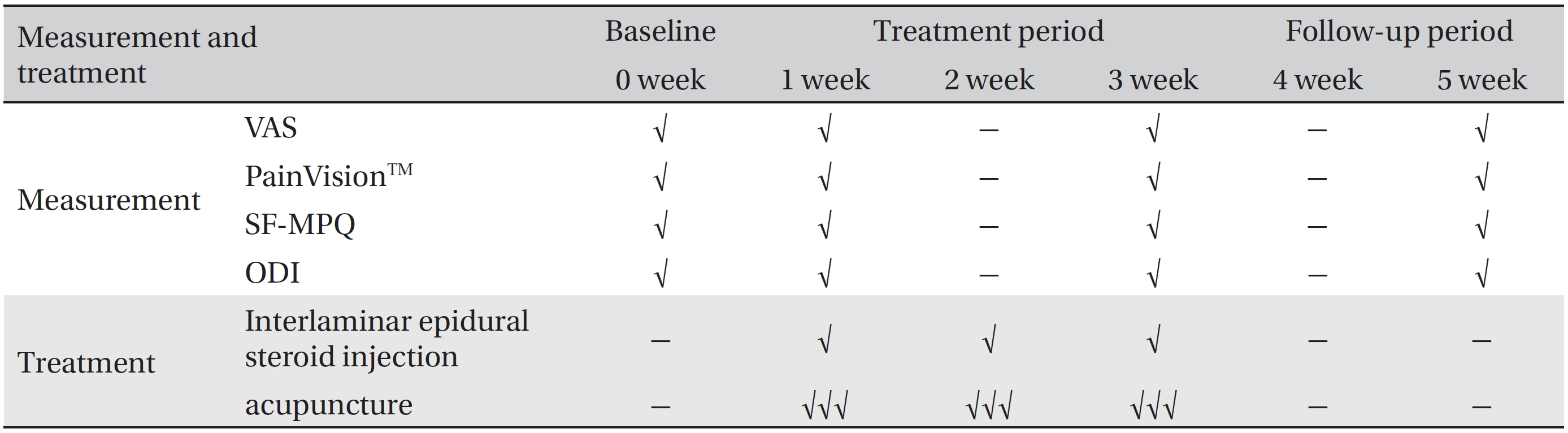
Schedule of treatments and outcome measurements throughout the 5-week pilot randomized controlled trial
The primary outcome measurements will be the VAS and PainVisionTM system scores. The secondary outcome measurements will be the answers on the short form- McGill pain questionnaire (SF-MPQ) and the oswestry disability index (ODI). Both the primary and the secondary outcomes will be measured at baseline and at one, three, and five weeks. The schedule of treatment and outcome assessments is presented in Table 1.
2.6.1. Primary outcome measurements
1) VAS: Pain intensity will be assessed using a 10 cm VAS of subjective pain assessment. Each patient will rate his or her pain on a scale of 0 - 10 (0, absence of pain; 10, the worst pain imaginable) [24, 25]. VAS measurements will be made at baseline, and at one, two, and three weeks.
2) PainVisionTM: PainVisionTM is a system used for the quantitative analysis of the perception and sensation of pain; it has recently been introduced in the field of pain and anesthesiology [26]. The PainVisionTM system consists of four devices: (1) the main PainVisionTM system unit, (2) a personal computer connected to the PainVisionTM system, (3) sensors, and (4) a printer. The specific protocol for using the system is as follows [27]: First, sensors that transmit an electric current will be attached to the right medial forearm. The current perception threshold that indicates the pain threshold of each subject will be measured three times, and the mean values will be used for analysis. Second, the pain compatible electrical current will be measured by gradually increasing the pulsed current applied to the right medial forearm. The pain due to the VCF and the magnitude of electric stimulation are believed to be equal. The pain compatible electrical current will be measured three times, and the mean values will be used for analysis. On the basis of these measurements, the pain intensity will be calculated using the following equation:
Pain intensity = 100 × (pain compatible electrical current - current perception threshold) / current perception threshold.
2.6.2. Secondary outcome measurements
1) SF-MPQ: The SF-MPQ, a shorter version of the McGill pain questionnaire (MPQ), is a multidimensional measure of perceived pain in adults with chronic pain [28, 29]. The main component of the SF-MPQ consists of 15 descriptors (11 sensory; 4 affective) from the original MPQ. The descriptors are rated on an intensity scale (0, none; 1, mild; 2, moderate; or 3, severe). The SF-MPQ also includes the present pain intensity (PPI) scale. The PPI scale is a measure of the magnitude of pain experienced by an individual and a numeric verbal combination that indicates overall pain intensity and includes six levels (0, none; 1, mild; 2, discomforting; 3, distressing; 4, horrible; and 5, excruciating) [30]. Higher numbers indicate more severe pain. The SF-MPQ will be measured at baseline and at one, three, and five weeks.
2) ODI: The ODI is one of the most frequently used tools for measuring disability related to low back pain [31]. The ODI contains 10 questions about daily activities, including inventories of pain intensity, personal care, lifting, walking, sitting, standing, sleeping, sexual life, social life, and traveling. Each question is rated on a scale from 0 to 5 points. The ODI scores range from 0 to 50. Higher scores indicate greater disability. The validated Korean version of the ODI [32] will be administered at baseline, and at one, three, and five weeks.
The safety of acupuncture will be determined by the red blood cell (RBC) count, hemoglobin level, platelet count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), hematocrit (HCT), total white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine level, serum sodium level, serum potassium level, and serum chloride level. All patients will be evaluated at two time points: at the screening visit and after treatment cessation.
Any reported adverse events will be recorded throughout the study, and vital signs will be monitored at each visit. The patients will be requested to voluntarily report information about adverse events, and the researcher will confirm the occurrence of adverse events through methods such as a medical interview. The details of the adverse events, such as the date of occurrence, degree of the adverse event, causal relationship with the treatment, other treatments or medications that are suspected to have caused the adverse event, and the treatment of the adverse event, will be recorded.
All patients will have the right to withdraw from the study at any time. Participation will be ended at any stage if the patient refuses to continue, withdraws his or her consent, or violates the inclusion or exclusion criteria or the trial protocol. The trial will be stopped if the principal investigator believes that an unacceptable risk of a serious adverse event exists.
The statistical significance level will be set at 5%, and the data will be processed with the last observation carried forward method for the intention to treat analysis. Statistical analysis in this study will be based on the Clinical Trial Statistics Guidelines (KFDA, 2000) and will be performed using IBM SPSS Win ver. 19.0 statistical software.
All demographic and clinical characteristics of the subjects (e.g., sex, age, and weight) will be processed based on descriptive analyses. Quantitative data will be presented as the average and standard deviation, the median value, and the range. Qualitative data will be presented as the frequency and the percentage. The study will identify the comparative equivalence of demographic variables and clinical characteristics between the experimental and the control groups.
In order to identify differences in VAS, PainVisionTM, SFMPQ and ODI scores between the experimental and the control group based on time (baseline, weeks one, three, and five), a repeated measure two factor analysis will be performed to identify differences between groups, differences within each group based on time, and the effects of the interactions of the variables based on group. If the interaction between group and time is statistically significant, the point at which the pattern of results between the two groups changes will be checked. The Chi square test will be used to compare groups and the incidence frequency of adverse events related to acupuncture and interlaminar epidural steroid injection.
Nonsurgical management is one of the preferred approaches for treating a VCF [33]. In general, appropriate analgesics should be prescribed to reduce pain and, thus, promote early mobilization [34]. In those who do not achieve acceptably rapid symptomatic relief with nonsurgical treatment, the judicious use of vertebral augmentation by vertebroplasty or kyphoplasty may provide more rapid pain relief. However, long-term outcomes have not been defined [7]. Traditional nonsteroidal anti-inflammatory medications are related to the risk of nausea, gastritis, ulcers, gastrointestinal bleeding, and renal insufficiency [35]. Narcotics have significant side effects, including reduced gastrointestinal motility, urinary retention, reduced respiratory drive and cognitive deficits related with loss of balance, increase in the number of falls and depression [36]. Vertebroplasty and kyphoplasty are invasive procedures that carry risks of symptomatic epidural cement leakage, causing nerve root injury (in 0.4% to 4% of patients) [37, 38], and symptomatic cement pulmonary embolism (in approximately 0.1% of patients) [38, 39]. More worrisome is the possibility that the procedures may increase the risk of a subsequent VCF by increasing the mechanical load on the vertebrae adjacent to those being treated [40]. Considering the high prevalence of VCFs in the general population and the side effect of the present treatment, a demand for a more effective and safe therapy exists.
Acupuncture, one of the most important parts of complementary and alternative medicine, has been used for thousand years. In Korea, many patients with a VCF are treated using traditional Korean medicine, including acupuncture. However, no study so far has investigated the efficacy and the safety of acupuncture for treating a VCF. A pilot, randomized, controlled trial will provide the feasibility and a clinical foundation for a future full scale trial. The outcomes will provide additional resources for incorporating acupuncture into existing treatments such as nonsteroidal anti-inflammatory medications, narcotics, and vertebral augmentation.