The purpose of the study is to assess both the approximate lethal dose and the single dose intramuscular injection toxicity of Danggui (Angelica gigantis radix) pharmacopuncture (DGP) in Sprague-Dawley (SD) rats.
The experiments were conducted at the good laboratory practice (GLP) laboratory, Biotoxtech Co., which is a laboratory approved by the ministry of food and drug safety (MFDS). The study was performed according to the GLP regulation and the toxicity test guidelines of the MFDS (2009) after approval of the institutional animal care and use committee of Biotoxtech. Single doses of DGP were injected intramuscularly into the rats in three test groups of 6 week old SD rats (5 male and 5 female rats per groups) in the amounts of 0.1, 0.5, and 1.0 mL/animal for groups 2, 3, and 4, respectively, and normal saline solution in the amount of 1.0 mL/animal was injected intramuscularly into the rats (5 male and 5 female rats) in the control group. Observations of the general symptoms and weight measurements were performed during the 14 day observation period after the injection. Hematologic and serum biochemical examination, necropsy, and a local tolerance test at the injection site were done after the observation period.
No death was observed in three test groups (0.1, 0.5 and 1.0 mL/animal group). In addition, the injection of DGP had no effect on general symptoms, weights, hematologic and serum biochemical examination, and necropsy. The results from the local tolerance tests at injection site showed no treatment related effects in the SD rats.
The results of single dose intramuscular injection of DGP suggest that the approximate lethal dose is above 1.0 mL/animal for both male and female SD rats and that intramuscular injection of DGP may be safe.
Pharmacopuncture is a unique acupuncture treatment of traditional Korean medicine (TKM) which combines acupuncture based on the meridian theory and herbal medicine based on Qi and flavor theory. Specific amounts of herbal extracts are injected at acupoints according to the differential diagnosis of a TKM doctor [1]. Danggui (DG) is commonly refers to Angelica plants that belong to the family of Apiaceae. The types of DG are
Danggui pharmacopuncture (DGP), namely AGR pharmacopuncture, is an acupuncture method in which an AGR extract solution is injected at an acupoint to treat disease. Experimental studies have reportedly shown that DGP has analgesic, antioxidant and potential chemopreventive effects [4-6] and that is has a beneficial effect on immune function, recovery anemia due to blood loss, liver protection against acute drug induced hepatitis and suppression of neuron cell regeneration in ischemic stroke or neural diseases [7-11]. Although research on the efficacy of DGP is ongoing, safety studies of DGP have not been reported. The toxicity of DGP administered by using intramuscular injection, which is the main treatment method of pharmacopuncture, needs to be determined. The purpose of this study is to assess the single dose intramuscular injection toxicity of DGP in Sprague-Dawley (SD) rats and to evaluate its approximate lethal dose.
One kg of ground
SD rats (Orientbio Inc., Korea), which are widely used for safety tests, were used in this study. Basic visual examinations were done when the animals were received, after which their weights were measured with an electronic scale (CP3202S, Sartorius, Germany). The animals were then moved to animal chambers. The general symptoms were observed daily during the 7-day stabilization period. The body weights, general basic symptoms, and weight changes were measured on the last day of stabilization in order to confirm the health status of the rats. At the time of intramuscular (IM) injection, the weight range of the 6 6-week-old male rats (n = 20) was 191.5 ─ 217.2 g and that of the 6-week-old female rats (n = 20) was 145.5 ─ 173.4 g.
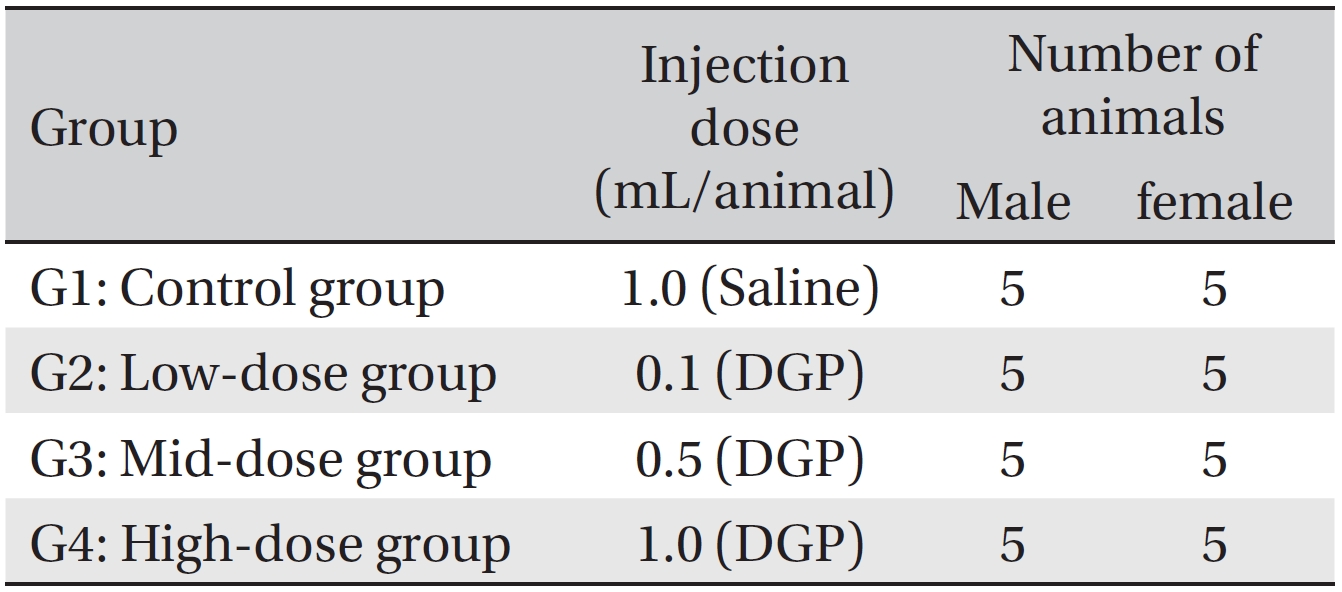
The animals were housed in a room maintained at a temperature of 21.0 ─ 23.2°C and a relative humidity of 40.9% ─ 59.4%, with artificial lighting from 07:00 to 19:00 (150 ─ 300 Lux) and a ventilation rate 10 ─ 15 times/hour. During the stabilization period, the animals (three during the stabilization period and one during the observation period) were housed in stainless steel wire mesh cages 260 mm (w) × 350 mm (d) × 210 mm (h) during the single dose intramuscular injection toxicity test. Animals were allowed free access to irradiation sterilized pellet feed (Teklad Certified Irradiated Global 18% Protein Rodent Diet, 2918C, Harlan Laboratories, Inc., USA). This study was conducted with the approval of the Institutional Animal Care and Use Committee of Biotoxtech Co. (Approval No: 110156) and was performed in compliance with the good laboratory practice (GLP) regulation and the toxicity test guidelines of the ministry of food and drug safety (MFDS). Grouping was done on the last day of stabilization. Twenty animals of each sex with the mean weight were selected and randomly placed into 4 groups with 5 male rats and 5 female rats for each group so as to have the same average weight per group (Table 1).
[Table. 1] Grouping of the animals
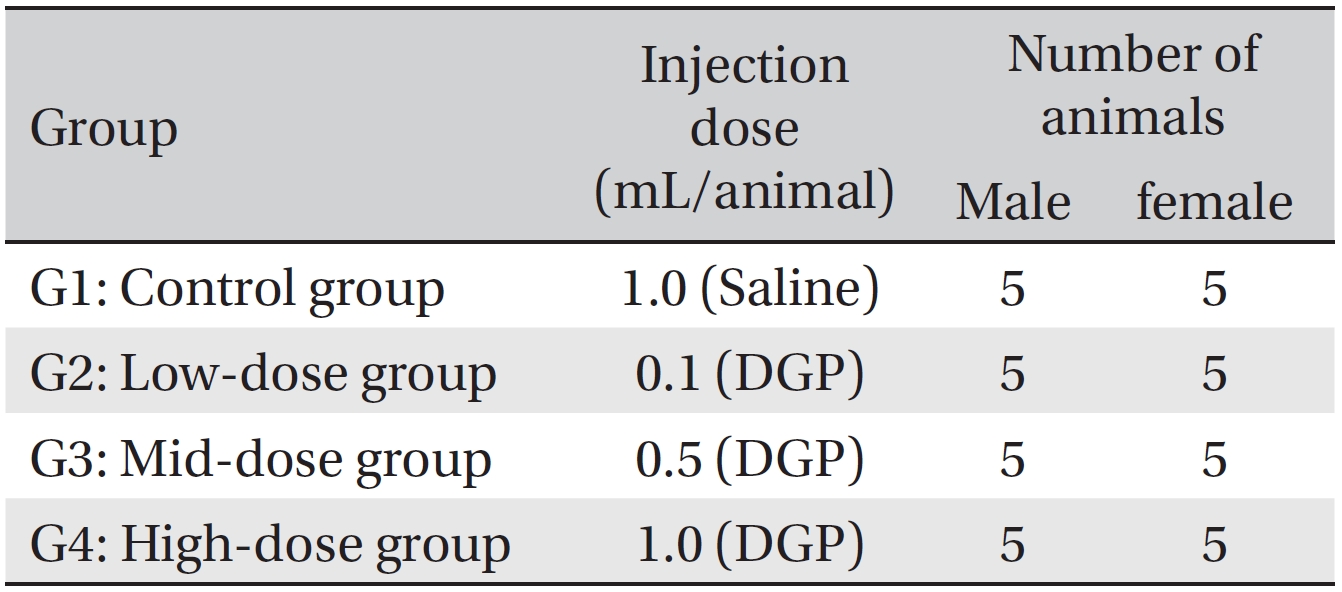
Grouping of the animals
DGP was injected intramuscularly by using a method applicable to clinics. Dosages for the control and the highdose groups were 1.0 mL/animal of saline and of DGP, respectively, and those for the low dose and the mid dose groups were 0.1 and 0.5 mL/animal, respectively. A single dose of DGP was injected into the left thigh muscle of the SD rats in the low and the mid dose groups, and 0.5 mL of DGP was injected into each thigh muscle of the SD rats in the control and the high dose groups. The injections were done using disposable syringes (1 mL, 26 gauge). The expected clinical dose of DGP is 1.0 mL/time. The results of a pilot test (Biotoxtech Study No.: B12875P) showed no death for a single dose intramuscular injection of 1.0 mL/ animal in both male and female rats. As a result, 1.0 mL/ animal was selected as the high dose, and 0.5 mL and 0.1 mL were set as the mid and the low doses. Normal saline (Choongwae Pharma Corp., Korea) was injected for control group. At the day of injection (day 0), clinical signs of toxicity, such as the onset time, the recovery time, etc., and any deaths were noted at 30 minutes, 1 hour, and 2 hours after the first injection, and at 30 minutes, 1, 2, 4 and 6 hours after the second injection. Clinical signs were observed daily for 14 day after injection. The weights of the SD rats were measured on the day of the injection (before injection), and on the third, seventh, and fourteenth (necropsy day) days after injection. Necropsy and blood collection were performed after fasting for more than 18 hours. Approximately 1 mL of blood was taken from the abdominal aorta by using a syringe needle under isoflurane anesthesia. The blood samples of all rats were collected in a bottle containing ethylenediaminetetraacetic acid (EDTA) and were analyzed using a blood counting analyzer (ADVIA 120, Siemens, Germany) to measure the red blood cell count (RBC), hemoglobin concentration (HGB), hematocrit (HCT), mean corpuscular cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular cell hemoglobin concentration (MCHC), platelet count (PLT), white blood cell count (WBC), WBC differential count (neutrophils, lymphocytes, monocytes, eosinophils, and basophils), and reticulocyte count. About 2.0 mL of the blood taken during necropsy was placed into a tube containing 3.2% sodium citrate, and plasma was obtained by using centrifugation (3000 rpm) for 10 minutes. The prothrombin time (PT) and the active partial thromboplastin time (APTT) were measured using an automated coagulation analyzer (Coapresta 2000, Sekisui, Japan).
Blood samples taken from the abdominal aorta were centrifuged at 3,000 rpm for 10 minutes and analyzed using an automatic analyzer (7180, Hitachi, Japan) and an electrolyte analyzer (AVL9181, Roche, Germany). Serum biochemistry parameters, including blood urea nitrogen (BUN), creatinine (Crea), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), total protein (TP), albumin (Alb) and albumin/globulin ratio (A/G ratio), total bilirubin (T-Bili), total cholesterol (T-Chol), triglycerides (TGs), phosphorus (P), glucose (Glu), calcium (Ca), sodium (Na), potassium (K) and chloride (Cl), were measured.
After the observation period, necropsy was conducted after all surviving animals had been euthanized by abdominal aorta bloodletting with carbon dioxide (CO2) gas anesthesia. Detailed visual inspections of the organs and tissues of all surviving animals were done. A local tolerance test was conducted on the tissue at the injection site for all animals. The tissue from the injection site was fixed with 10% neutral buffered formalin solution. The fixed tissues were routinely processed, embedded in paraffin, and sectioned. The sections were stained with hematoxylin and eosin stain for microscopic examination.
Weight, hematology and serum biochemistry data were analyzed by using statistical analysis system (version 9.3, SAS Institute Inc., USA). Data are presented as mean ± standard deviation. The test for equality variance was the Bartlett test (
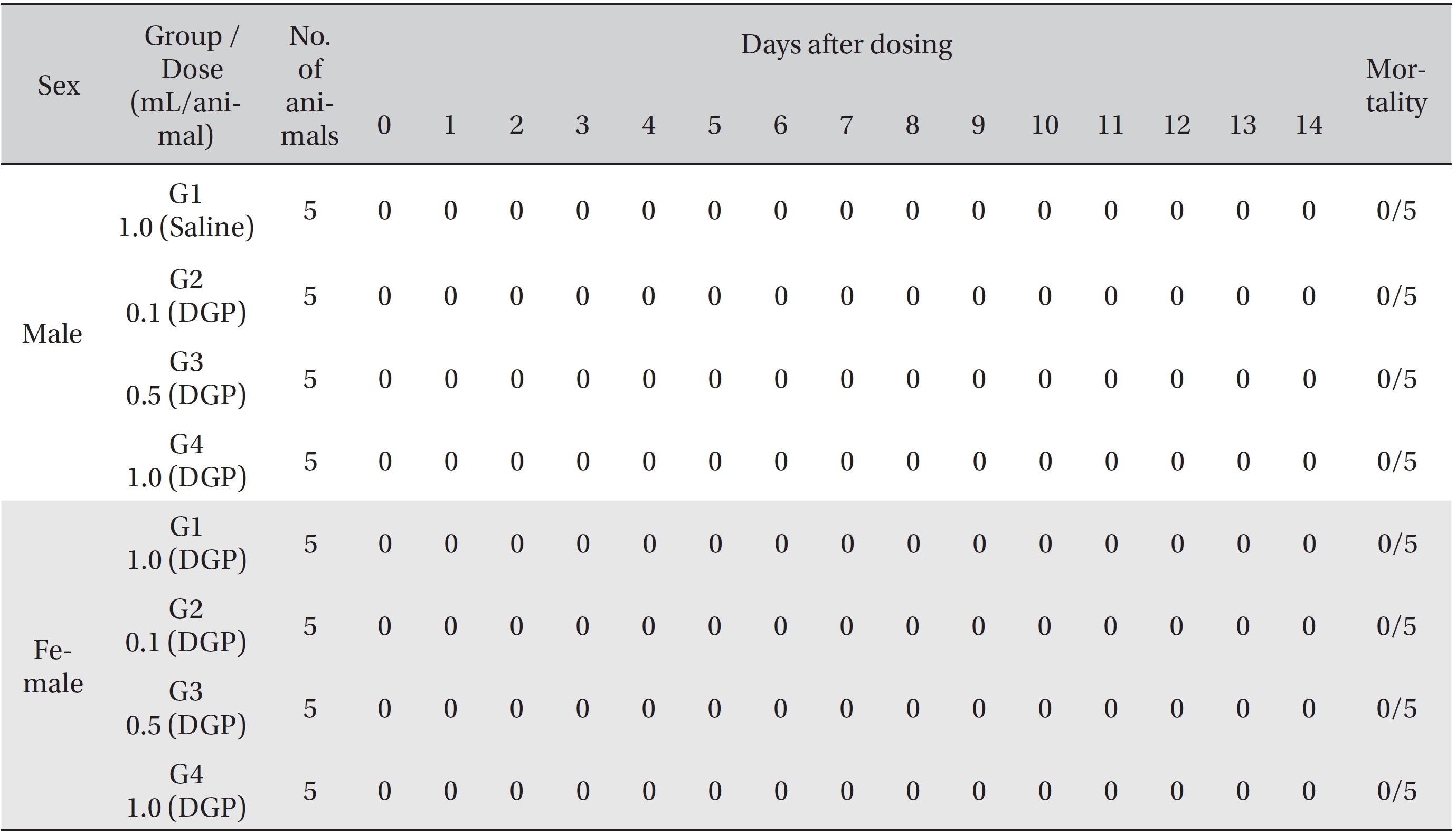
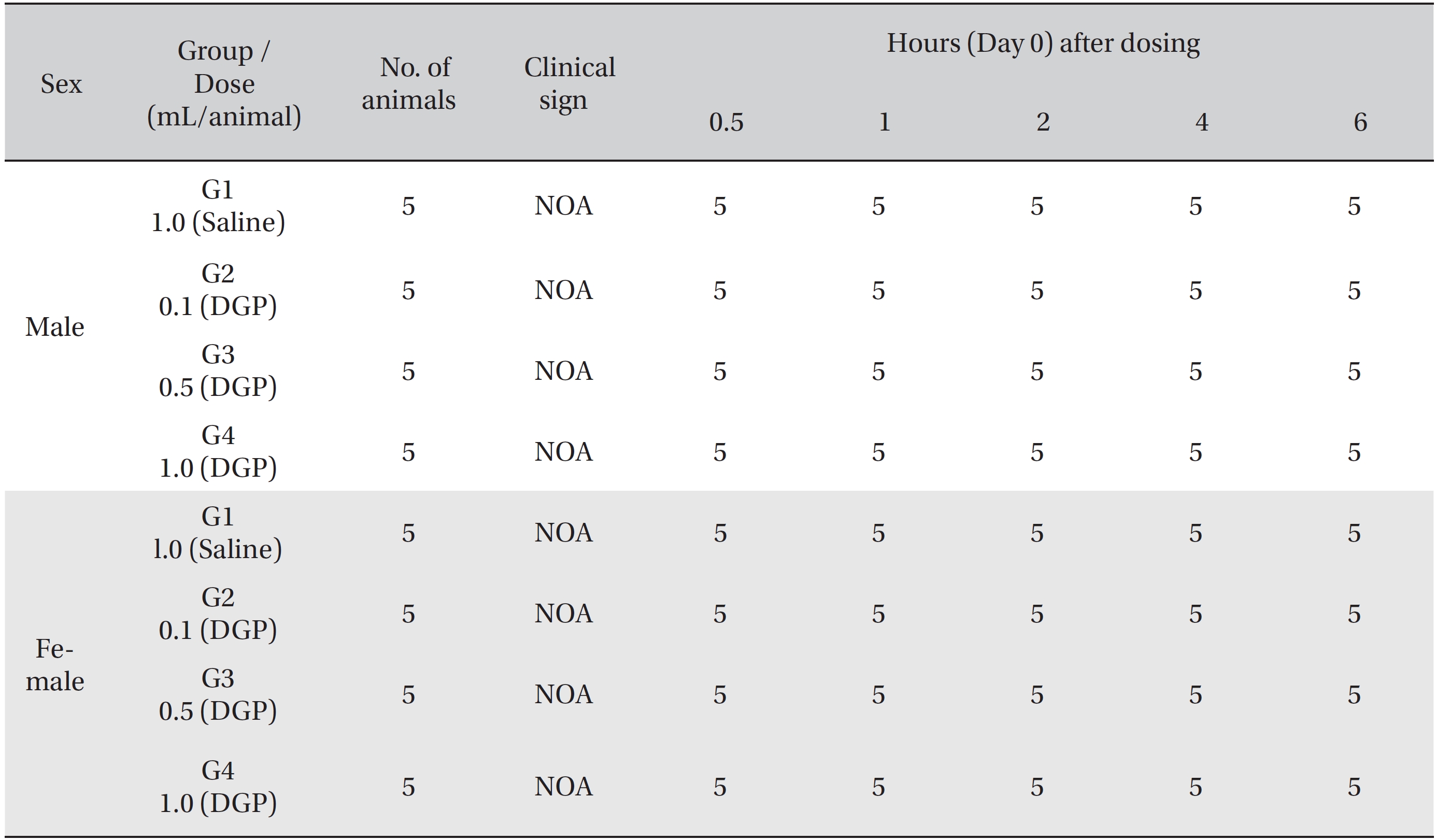
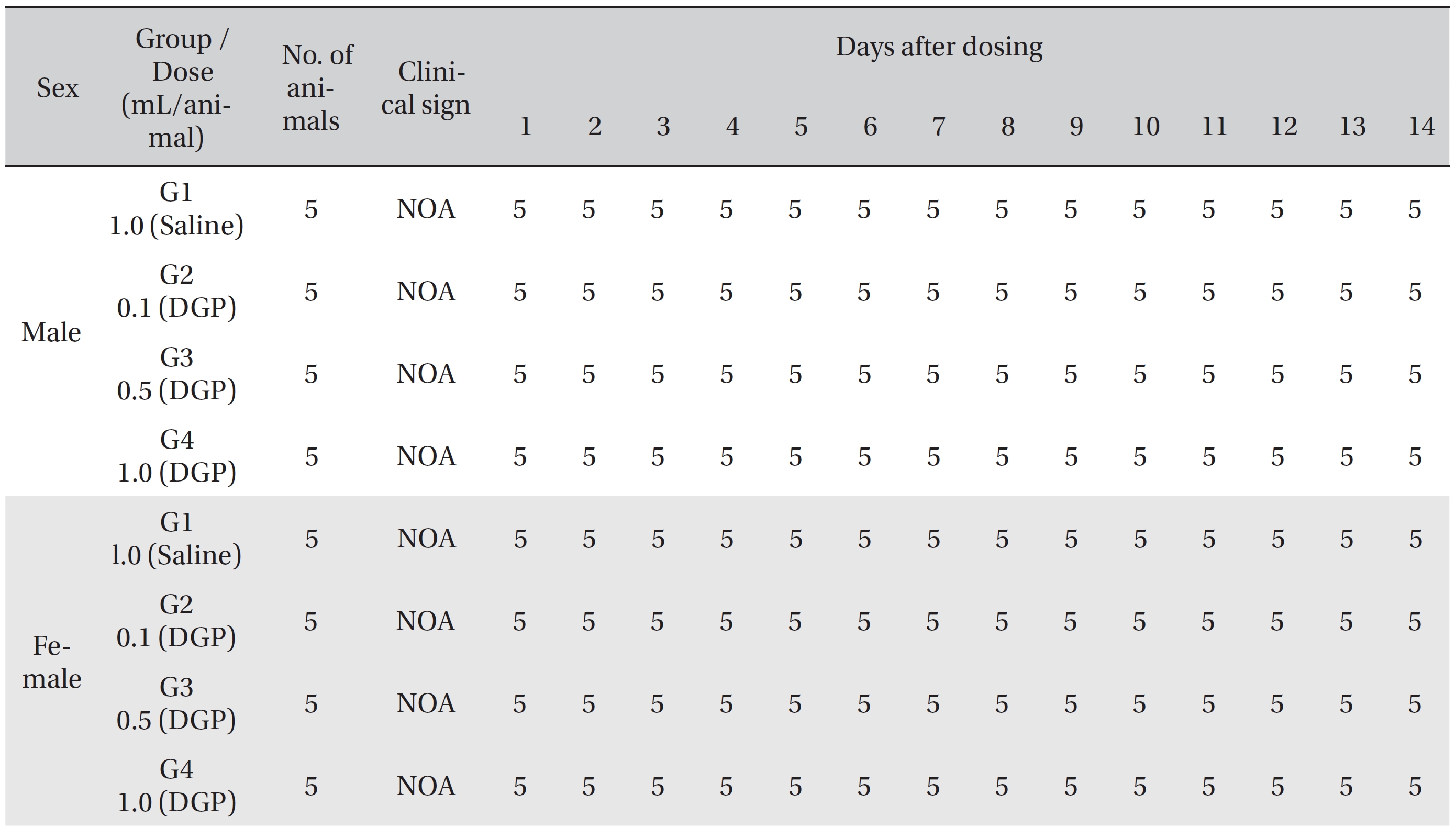
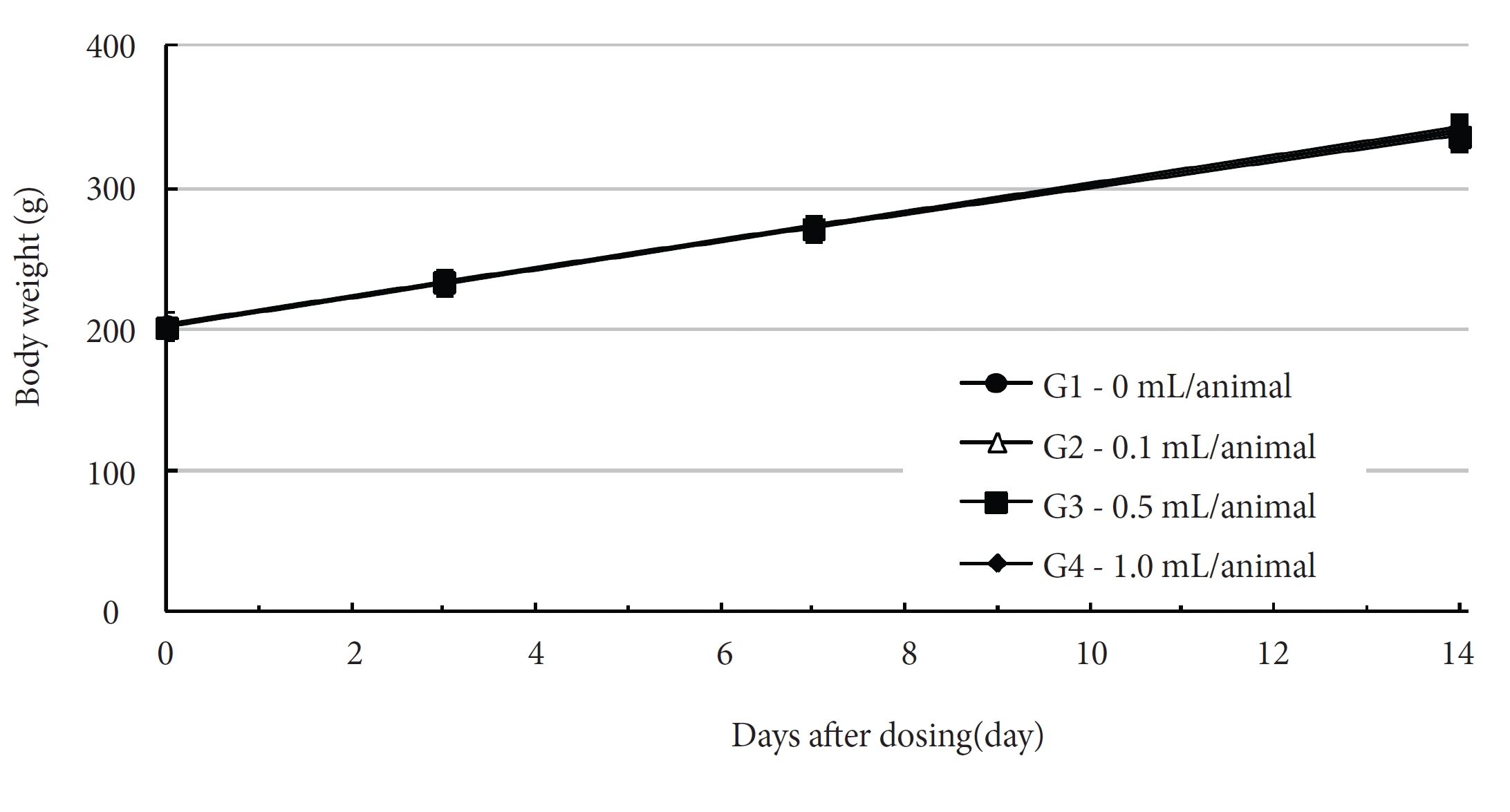
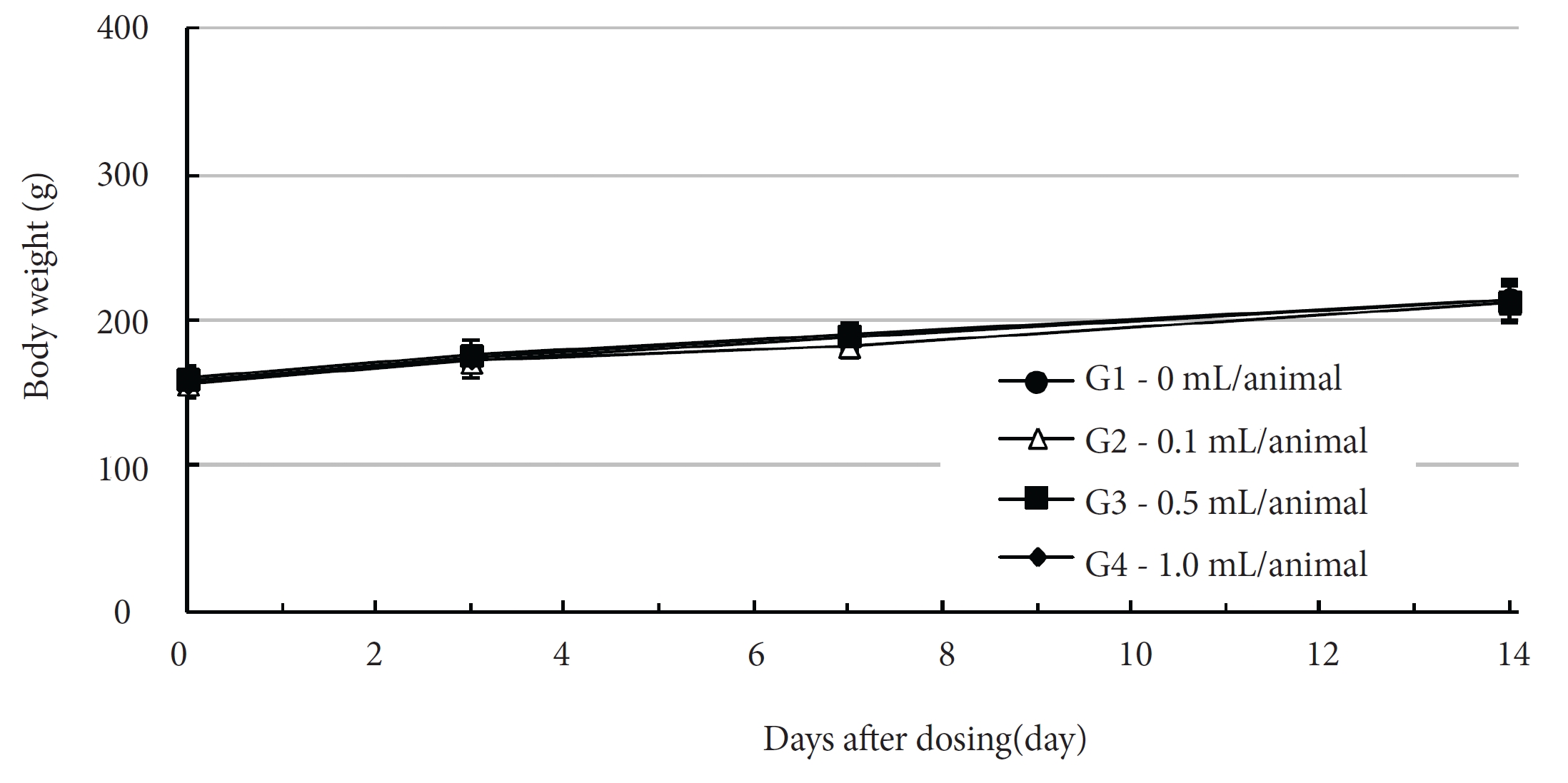
No deaths of both male and female rats were observed in any of the four groups during the observation period (Table 2). In addition, no clinical signs were observed in either male or female SD rats in the control group or in the three test groups during the observation period (Tables 3, 4). Furthermore, no statistically significant changes in weight were observed for either male or female SD rats in the test groups compared with those in the control group during the observation period (Figs. 1,2).
No significant changes in any of the hematological parameters, such as RBC, HGB, HCT, MCV, MCH, MCHC, PLT, WBC, WBC differential count (neutrophils, lymphocytes, monocytes, eosinophils, and basophils), and reticulocyte count, were observed in any of the groups. In addition, no meaningful changes in serum biochemistry, such as BUN, Crea, ALT, ALP, GGT, TP, Alb, A/G ratio, T-Bili, T-Chol, TGs, P, Glu, Ca, Na, K, and Cl, were found in any of the groups. Significant changes of parameters among the test groups were minimal. No dose dependent toxicological signification was observed in any of the groups, and no macroscopic abnormalities were observed at necropsy in either both male and female SD rats in the control and the test groups during the observation period (Table 5).
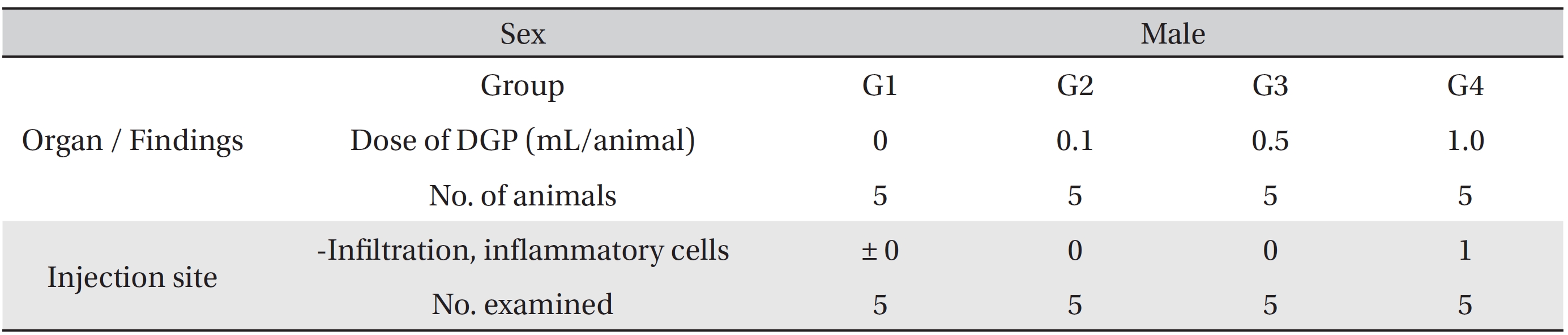
Local tolerance tests at the injection sites of all animals showed no treatment-related effects. Moderate linear infiltration of inflammatory cells at the injection site was found in a male rat from the 1.0 mL/animal dose test group. Minimal infiltration was observed in a female rat from both the 0.5 mL and 1.0 mL/animal dose test groups. All sites of infiltration were confined to biceps femoris region (Table 6).
DG contains essential oil and water-soluble materials. The main constituents of essential oil are ligustilide (45%), flavonoids, carvacrol, and n-butylidene phthalide. The water soluble materials are composed of mainly ferulic acid (0.094%) with nicotinic acid, uracil, adenine, and polysaccharide. In terms of pharmacological aspects, organic acids and phthalide are active components of DG. Phthalide is a lactone that has a cyclic structure with a ketone body, and various forms of phthalides exist in DG, such as n-butylidenephthalide, ligustilide, and n-butylphthalide. Phthalide is a volatile oil, and the odor of DG results from ligustilide and n-butylidenephthalide. In addition, coumarin derivatives such as oxypeucedanin, osthole, imperatorin, psoralen, and bergapten are included [2-3, 12].
The characteristics of DGP are as follows: As the medicinal effects of DGP depend on the essential oils, such as ligustilide and the water soluble constituent, the extracted essential oil components of DGP are micro pneumatized to be water soluble to penetrate easily throughout the body. Therefore, this enables DGP to contain as much of both the water soluble materials and the essential oils as possible to maximize its efficacy.
[Table. 2] Summary of mortality
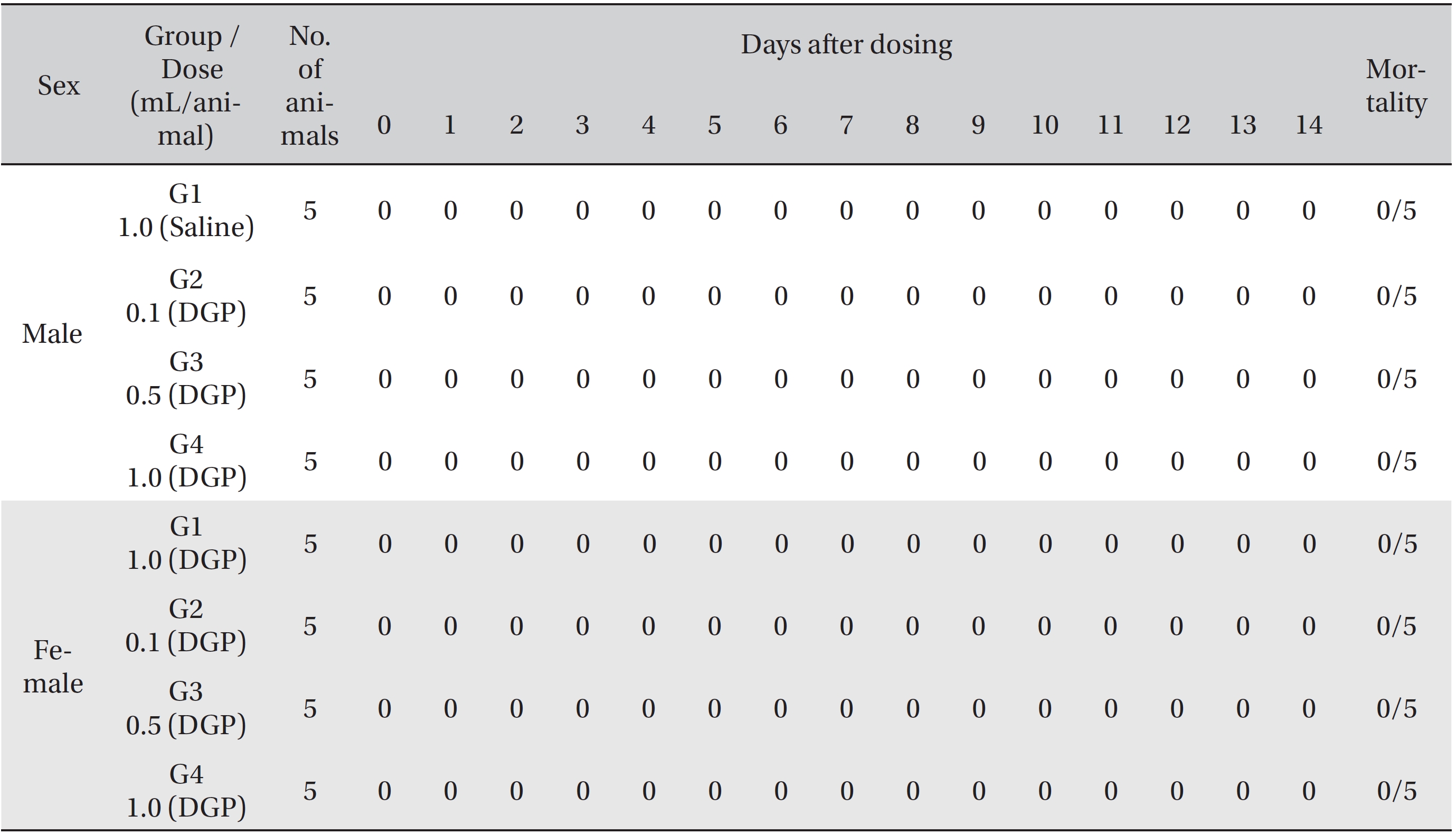
Summary of mortality
[Table. 3] Summary of clinical signs by hour after dosing
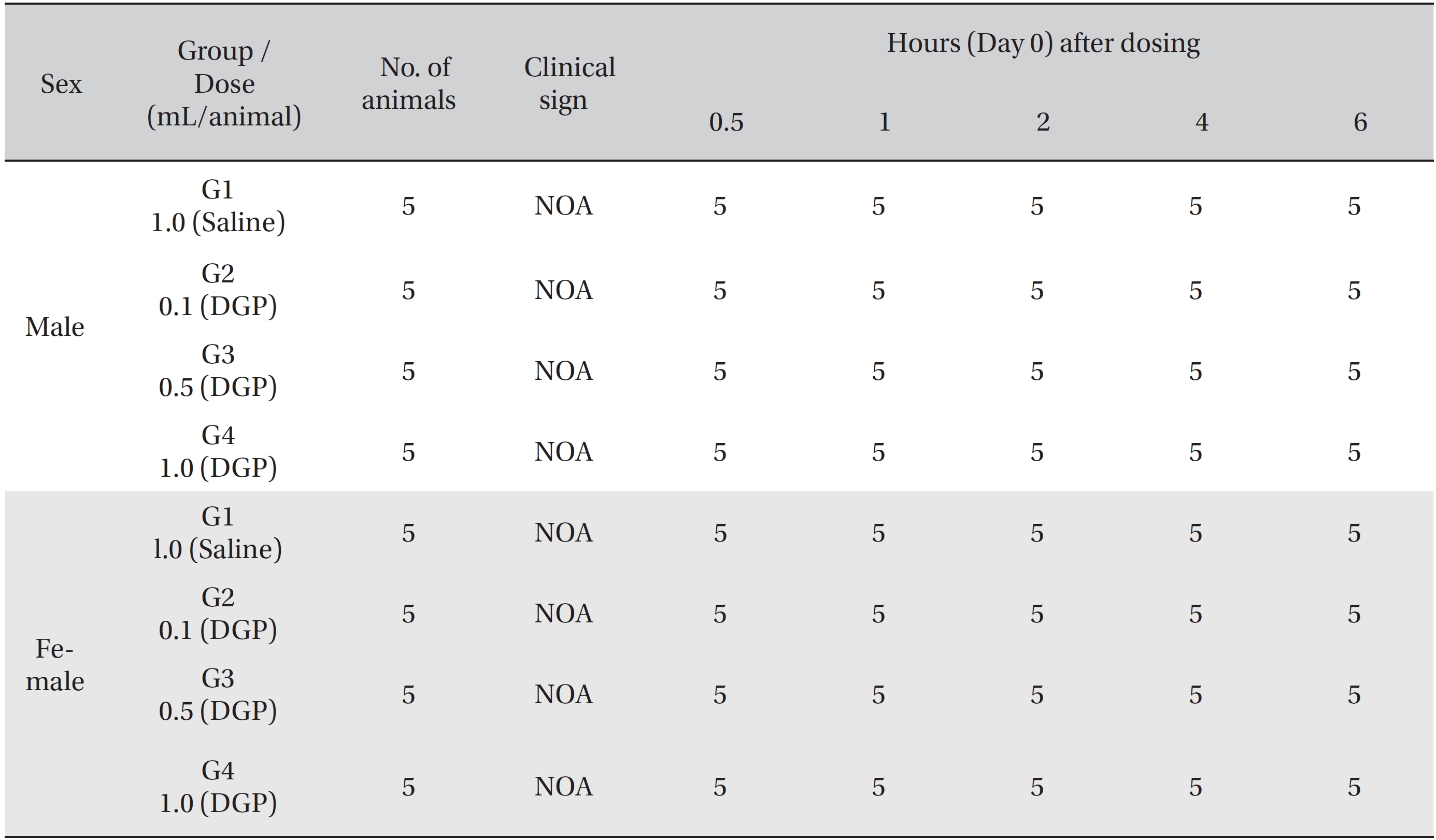
Summary of clinical signs by hour after dosing
[Table. 4] Summary of clinical signs by day after dosing
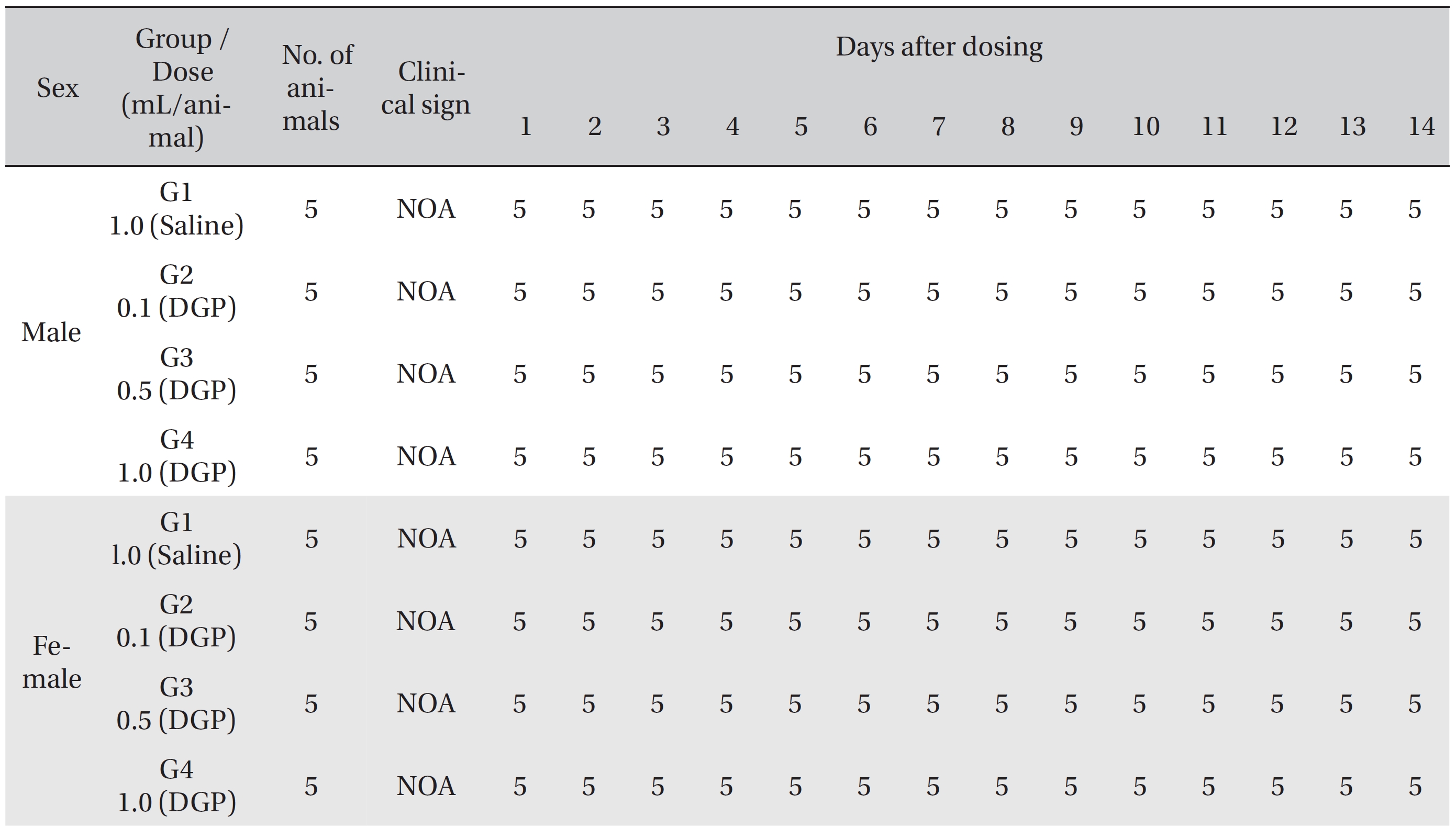
Summary of clinical signs by day after dosing
[Table. 5] Summary of necropsy findings
Summary of necropsy findings
[Table. 6] Summary of histopathological findings.
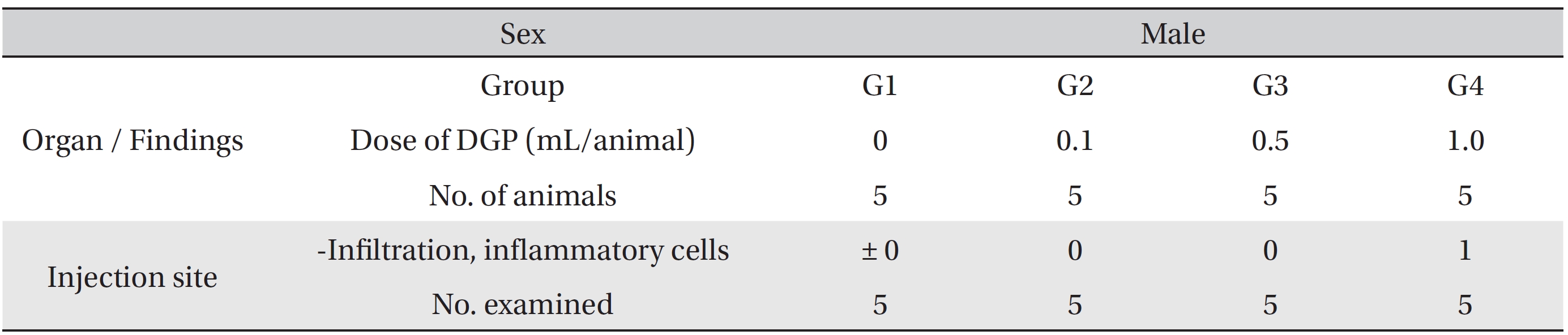
Summary of histopathological findings.
DG is a representative medicinal herb for treating blood deficiency. The main effects of DG are blood related actions, such as inhibition of platelet aggregation and the antithrombotic effect. It also has hematogenetic effects that stimulate hemopoietic stem cells and colony forming unit-granulocyte monocyte (CFU-GM) [3, 8]. DG also contains components that both stimulate and inhibit the smooth muscles of the uterus through bi-modal functions that mediate uterine diseases, such as dysmenorrhea, irregular menstruation, oxytocic uterine contractions, and uterine hemorrhage [3, 13]. The water extract of DG and ferulic acid release myocardial ischema, and ligustilide and n-butylidenephthalide of DG vasodilate and lowers blood pressure, which may have an effect when used to treat cardiovascular diseases [3, 13]. Based on the effects of DG described above, DGP can be used to treat gynecological diseases, such as dysmenorrhea, as well as cardiovascular diseases and hematologic diseases, such as stroke, arteriosclerosis, and cerebral thrombosis [10, 14-15].
Ligustilide of DG is a potent, toxic metabolite, as well as an active component. Furthermore, caffeic acid is contained not only in DG but also in other various plants. Despite its pharmacological effects, such as anti-carcinogenic, anti-oxidant, immune regulating, and anti-inflammation effects, carcinogenic properties of active metabolites have been reported, so the possibility of toxicity cannot be ruled out [16, 17]. However, no toxic results were observed in our single dose intramuscular injection toxicity tests. Moderate linear infiltration of inflammatory cells at the injection site was found on the histopathologic test, but seemed to have been the result of a needle injury rather than DGP injection. On the basis of our test results, a single dose intramuscular injection of DGP is safe. However, repeated toxicity tests are required to increase confidence in these safety results.
The results of single dose intramuscular injection of DGP suggest that the approximate lethal dose is higher than 1.0 mL/animal for both male and female SD rats and that intramuscular injection of DGP may be safe.