Metal contamination is considered to be one of the most ubiquitous and complex environmental issues today. Accumulation of heavy metals in soils and water is of particularly important because it can impact upon human health through possible contamination of food (Sadon et al., 2012). With the increasing demand for water for agricultural, domestic, industrial, and recreational purposes, remediation and reuse of contaminated waters receive prime attention globally (Opeolu and Fatoki, 2012). Different technologies, such as adsorption, chemical precipitation, coagulation/ flocculation, evaporation, complexation, membrane filtration, biological operations, electrochemical operations, ion exchange/solvent extraction, etc., have been employed in removing metals from contaminated water and wastewater. Due to the inherent merits such as easy handling, minimal sludge production, and regeneration capability, etc., associated with adsorption, it has been widely used as an efficient and effective technique for heavy metal removal (Chiban et al., 2012; Pagnanelli et al., 2003). However, the success of this technique is heavily dependent upon the nature of the adsorbent material used for the process. Recently, activated carbon is extensively used as the adsorbent, though the cost involved with the initial establishment and the regeneration system is relatively high (Aktas and Cecen, 2007). Therefore, the search for economically affordable alternatives remains one of the key priorities among researches.
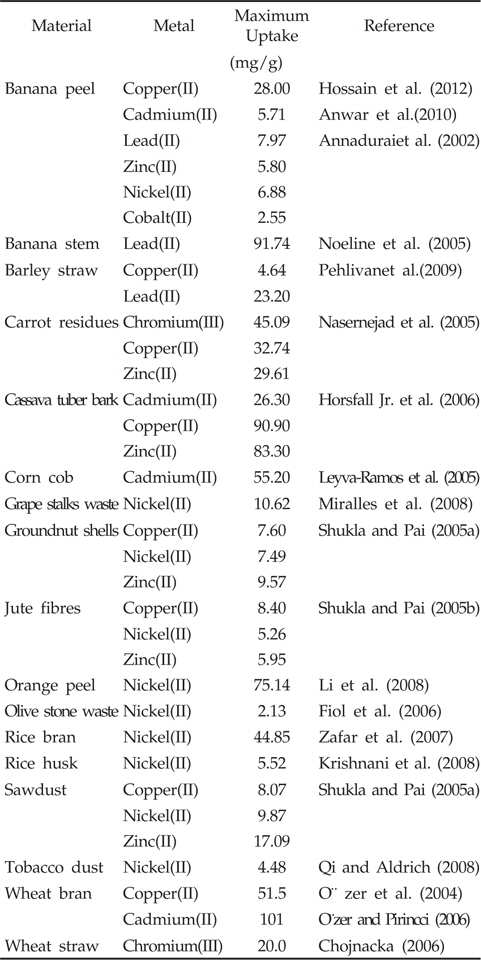
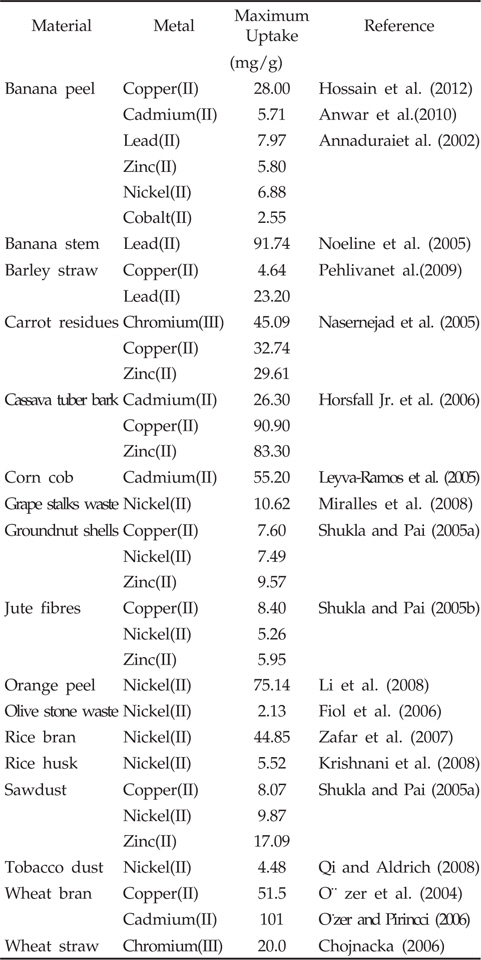
In this context, agricultural waste materials have been recognized as possible alternative adsorbents mainly due to the fact that they are easily available, cost-effective and highly efficient in adsorbing different kind of heavy metal ions (Montanher et al., 2005; Nasernejad et al., 2005). In fact, certain widely available plant materials remain unused resources, thus the use of them could indeed be an environmentally friendly approach too (Ashraf et al., 2011). Various plant-based adsorbent agents have been tested under different conditions, such as seaweed (Basha et al., 2008; Hashim and Chu, 2004), marine algal biomass (Sheng et al., 2004), seafood processing waste sludge (Lee and Davis, 2001), sugar beet pulp (Reddad et al., 2002b), banana peel (Ashraf et al., 2011; Castro et al., 2011; Hossain et al., 2012), papaya wood (Saeed et al., 2005), grape stalk wastes (Villaescusa et al., 2004), neem bark (King et al., 2007), neem biomass (Arshad et al., 2008), tea-waste (Malkoc and Nuhoglu, 2007; Kamsonlian et al., 2011a), sawdust (Garg et al., 2004; Memon et al., 2005), tamarind bark (Prasad and Abdulsalam, 2009), tamarind hull (Verma et al., 2006), potato peel waste (Ahalya et al., 2005), maize corn cob, jatropha oil cake, sugarcane bagasse (Garg et al., 2007), rice husk (Kumar and Bandyopadhyay, 2006), rice straw (Gao et al., 2008), wheat straw (Robinson et al., 2002), wheat shells (Bulut et al., 2007), wheat bran (Sulak et al., 2006), maize leaf (Babarinde et al., 2006), wood and bark (Mohan et al., 2007), teak leaf powder (King et al., 2006), rubber leaf powder (Hanafiah et al., 2006), pine bark (Al-Asheh et al., 2000), saltbush (Sawalha et al., 2005), and olive pomace (Pagnanelli et al., 2003), orange peel (Kamsonlian et al., 2011b), etc. By contrast, many crop residues are reported to be excellent adsorbents (Table 1) with good adsorption capacity better than activated carbons and commercial ion exchangers. However, the use of them in their original forms is not yet fully exploited (Anirudhan and Shibi, 2007). Moreover, many of the studies have been conducted with particular type of wastes with specific aqueous-phase applications. The present paper reviews literature dedicated to use of banana peel in removal of heavy metals from waters.
Banana is one of the most popular fruit crops grown in more than 130 countries, particular in tropical and sub-tropical regions of the world. The crop is grown by small-and large-scale producers alike and the annual world banana production exceeds 100 million tons (UNCTAD, 2012). Though the vast majority (over 90%) of the production is auto-consumed, a portion worth about $ 8 billion is still traded internationally, which shows an almost steady annual growth rate (UNCTAD, 2012).
Banana peel, which represents about 40% of total weight of the fresh fruit (Anhwange et al., 2008) is generally considered to be a waste material. As of the production records mentioned above, it is apparent that the banana industry produces more than 40 million tons of banana peel annually (peel represents about 40% of the total weight of fresh banana). Exploring alternative uses of banana peel would thus bring an additional value to the industry.
Fruit peels generally contain organic compounds such as cellulose, hemicellulose, pectin substances, chlorophyll pigments, and some other low molecular weight compounds (Xiaomin et al., 2007). The pectin substances, a complex heteropolysaccharides containing galacturonic acid, arabinose, galactose, and rhamnose, are found in fruit peels (Reddad et al., 2002a). Galacturonic acid with the carboxyl functions could make pectin substances a strong metal adsorbent in aqueous solutions (Saeed et al., 2005). As reported by Mohapatra et al. (2010), banana peel is found to be a good source of pectin (10-21%), lignin (6-12%), cellulose (7.6-9.6%), hemicelluloses (6.4-9.4%), and galactouroninc acid. Furthermore the pectin extracted from banana peel also contains glucose, galactose, arabinose, rhamnose, and xylose (Emaga et al., 2008).
Under this background, banana peel is recognized to be an economically viable and environmentally sound adsorbent for removal of heavy metals from contaminated waters. According to Hossain et al. (2012), 1g of banana peel can adsorb 28 mg of Cu2+ in a favourable condition, and thus provides with a potential alternative of Cu2+ removal from water. Moreover, it could adsorb 5.71 and 2.18 mg/g of Cd2+ and Pb2+, respectively (Anwar et al., 2010). Annadurai et al. (2002) studied the adsorption capacity of divalent heavy metal ions (Cu2+, Zn2+, Co2+, Ni2+ and Pb2+) onto acid (HNO3) and alkali (NaOH) treated banana peels. According to them, the adsorption capacity was in the order of Pb2+ > Ni2+ > Zn2+ > Cu2+ > Co2+. The maximum metal adsorptions were recorded as 7.97 (Pb2+), 6.88 (Ni2+), 5.80 (Zn2+), 4.75 (Cu2+), and 2.55 mg/g (Co2+). Memon et al. (2008b) assessed the practical applicability of banana peel to remove arsenic from contaminated water samples collected from 8 different areas of Sindh, Pakistan. They collected 100 mL of water samples, filtered and mixed with the banana peel before being shaken for 30 min. The contents of arsenic in the solution were measured before and after adsorption and results revealed that 98-100% of arsenic can be removed successfully using the banana peel. However, successful metal removal can be achieved only if due attention is paid on the followings.
The pH of the medium is considered to be a key factor governing the process of metal adsorption from an aqueous solution (Shafaghat et al., 2012). The solution pH is capable of influencing the dissociation state of the adsorbents, ionic state of functional groups and species of metals (Hossain et al., 2012). As reported by Fiol et al. (2006), the effect of pH on solution chemistry of the target metal includes hydrolysis, complexation by organic and/or inorganic ligands and redox potentials. At elevated pH values, adsorption is reduced due to high precipitation, ion exchange and aqueous metal hydroxide formation (Shafaghat et al., 2012). Furthermore, under basic condition, the binding sites may not activate as in acidic medium (Memon et al., 2008a). Analogous to these reports, Demirbas et al. (2009) reported that at high pH, copper began to precipitate as Cu(OH)2 +, resulting in lower adsorption. Hossain et al. (2012) also observed gradual reduction in Cu2+ adsorption onto banana peel when solution pH increased. According to them, Cu2+ adsorption capacity was declined when pH decreased below 2.0. However, it was reported to increase with the increase in pH from 2.0 to 6.0 (Hossain et al., 2012). As observed by Wang and Qin (2005), under slightly acidic conditions, Cu2+ is the dominant free species involved in adsorption. In fact, at slightly acidic conditions, linked H+ is released from the active sites allowing metals ions to be absorbed. However, when pH was further lowered, H3O+ ions begin to compete with Cu2+ for binding onto adsorbent sites resulting in poor adsorption capacity (Demirbas et al., 2009). Similar observations were made by Kaewsarn et al. (2008) who studied the Cd2+ biosorption onto banana peel. According to them, though the biosorption at very highly acidic condition (pH 1.0) was negligible, removal of Cd2+ increased with increasing pH from 1.0 to 5.0. In this regard, Shafaghat et al. (2012) reported that under highly acidic conditions, formation of links between metal ions and the active site is restricted by the excessive protonation of the active sites which ultimately results in lower adsorption. However, contrary to this Memon et al. (2008b) reported that adsorption of arsenic species is independent of pH, which of course raises the practical applicability of banana peel to remove arsenic from contaminated waters.
Hossain et al. (2012), based on the results of their study, reported that Cu2+ adsorption onto banana peel was relatively quicker than those reported for some other bio-adsorbents. They observed a rapid adsorption during the first 30 min, followed by a constant rate of Cu2+ removal for further 30 min before reached the equilibrium. In the case of arsenic, Memon et al. (2008b) reported that the equilibrium was established within 30 min, and 75% and 95% sorption was recorded for As(III) and As(V), respectively. According to them, no significant increase in the percent sorption onto banana peel was observed after 30 min. When particles of banana peel were introduced to the solution, metal ions could find flurry of vacant active binding sites resulting in rapid initial adsorption. This was further confirmed by Shafaghat et al. (2012), who studied the adsorption of Pb2+, Cu2+, and Cr3+ by five plant materials. According to them, the maximum removal of three metals was attained after a shaking period of 90 min. Once the majority of binding sites were occupied, formation of repulsive forces between the metal ions on the solid surface and the liquid phase makes it difficult for further binding to the remaining vacant surface sites (Achak et al., 2009; Srivastava et al., 2006). Thus, the adsorption of metal ions gradually decreases as time progressed. In fact, in order to bind, the metal ions have to pass through the deeper surface of the pores, which of course encounter substantial resistance leading to deceased adsorption during the later phase of adsorption (Srivastava et al., 2006). As stated by Shafaghat et al. (2012), the transportation rate of ions from the exterior to the interior sites of the adsorbent particles actually determines the adsorption rate of later phase.
It is well understood that the amount of metal removal is vastly dependent upon the metal concentration in the solution. As reported by Chojnacka (2006), who studied the effect of initial concentration of Cr3+ on adsorption by wheat straw, the adsorption rate was increased with the increase in the initial metal ion concentration. However, if the amount of biomass remains constant in the system, the metal removal efficiency may be reduced regardless the increased metal concentration (Zhou et al., 2007). Furthermore, Hossain et al. (2012) who studied the Cu2+ adsorption by banana peel reported that the adsorbent dose is also decisive for metal removal. They observed the highest Cu2+ removal (88%) when the initial Cu2+ concentration of 10 mg/L with the adsorbent dose of 5 g/L. Similar investigation was conducted by Anwar et al. (2010) to study the effect of adsorbent dosage on the adsorption of Cd2+ and Pb2+. They used different dosages of banana peel ranging 10-90 g/L, and the maximum removal was observed at the doses of 30 and 40 g/L, respectively, for Cd2+ (89.2%) and Pb2+ (85.3%). At the high doses of adsorbent, removal of metal may be affected by the partial aggregation among the available active binding sites (Anwar et al., 2010), whereas at low doses, lack of active binding sites may result in lower rate of metal removal (Karthikeyan et al., 2007).
Kaewsarn et al. (2008) used dried biosorbent derived from banana peel to study the removal of Cd2+ from aqueous solution and reported that particle size of banana peel had no effect on the removal of metal ions, which is analogous to Volesky (2003) who studied the effect of particle size on uranium and cadmium biosorption by Sargassum biomass. They used three sizes of biomass (0.5-0.7 mm, 1.0-1.4 mm and native whole seaweed for uranium; 0.5-0.7 mm, 0.84-1.00 mm and 1.0-1.4 mm for cadmium). According to them, no significant difference in sorption rate for the metals among the tested particle sizes was observed. Cossich et al. (2002), based on their finding with biosorption of chromium onto Sargassum biomass also reported that the rate of biosorption was independent from the particle size of the biomass. Kaewsarn et al. (2008) further stated that the higher biosorption level achieved by smaller particle size of the biosorbents may not be connected to the fact that smaller particle sizes give large surface areas. However, adsorption is considered to be a surface phenomenon; thus sorbents with higher surface area should exhibit a rapid adsorption than that of sorbents with lower surface area. Furthermore, as stated by Daifullah et al. (2004), the smaller particles do have higher specific surface area and low mass transfer resistance than the larger biosorbents. In fact, Daifullah et al. (2004) observed higher adsorption rate and uptake from the smaller and fine particles of the activated carbon adsorbent prepared from biomass than the larger particles of the same adsorbent. Demirbas et al. (2004) also have reported that the equilibrium might be achieved quickly if smaller particles were used. According to Park et al. (2010), smaller particles are better suited for a batch type removal setup due to the higher surface area of the biosorbent, however, not for column processes due to its low mechanical strength and clogging of the column. A wide range of particle sizes including 5 x 5 mm, 3 x 3 mm, 2 x 2 mm, 1 x1 mm, fine powder (<150 μm), semi-fine powder (150-300 μm), middle size powder (300-420 μm), semi-coarse size powder (420-600 μm), and coarse powder (600-900 μm) was used to study the effect of particle size of banana peel on the adsorption of Cu2+ (Liu et al., 2012). Based on the findings, they claimed that Cu2+ removal efficiency of powder particles is much higher than that of in larger sized particles. However, they observed no significant differences among different sized powder particles. As reported by Kaewsarn et al. (2008), ground particles irrespectively their sizes, make similar thickness of adsorbents, thus the effect of particle size was hard to observe. Contradicted reports on the effect of particle size might be due to the different experimental conditions under which above investigations have been conducted.
Hossain et al. (2012) studied the effect of shaking speed (30-200 rpm) on the adsorption of Cu2+ by banana peel. Both at low and high speeds, the rate of Cu2+ adsorption decreased and the maximum adsorption (88%) was recorded at near 120 rpm. At low speed, particles could not spread adequately for providing active binding sites for adsorption of metal, while at high speed, vigorously spreading the particles did not allow sufficient time to bind with metal ions (Anwar et al., 2010).
Banana peel has to be dried before being used as adsorbent. The effect of drying temperature on the metal removal efficiency was tested with various drying temperatures (30, 60, 90, 105, 120, and 150℃) (Liu et al., 2012). Based on the results, they reported that banana peel dried at higher temperature is more effective in removing Cu2+ than those dried at lower temperatures. As a possible explanation, they claimed that higher temperature could change the interaction area between the ligands on the cell wall and the metal ions through expanding the surface area of the adsorbents. However, taking the energy consumption for drying also into account, they suggested 120℃ as the most reasonable temperature for drying banana peels.
Based on the scale of production, banana peel could certainly be recognized as a promising crop-based material available for exploring alternative uses. The proven metal removal capacity of banana peel provides with a favorable platform to researchers to work on and to come out with a sound technology applicable in removal and recovery of metals from water and wastewaters. Such a green technology would not only address the much needed sustainable tool for cleaning contaminated waters, but of course bring an additional value to banana peel enhancing the fruit industry worldwide.