This study was performed to develop a single residue analytical method for new herbicide metazosulfuron in crops.
Brown rice, apple, mandarin, Kimchi cabbage and soybean were selected as representative crops, and clean-up system, partition solvent and extraction solvent were optimized. Instrumental limit of quantitation (ILOQ), linearity of calibration curve and method limit of quantitation (MLOQ) were determined based on the chromatography and whole procedures. For recovery tests, brown rice, apple, mandarin, Kimchi cabbage and soybean samples were macerated and fortified with metazosulfuron standard solution at three levels (MLOQ, 10 MLOQ and 100 MLOQ). And then those were extracted with acetonitrile, concentrated, and partitioned with ethyl acetate. Then the extracts were concentrated again and cleaned-up through NH2 (aminopropyl) SPE cartridge with acetone : dichloromethane (1% acetic acid) (20 : 80, v/v) before concentration and analysis with HPLC.
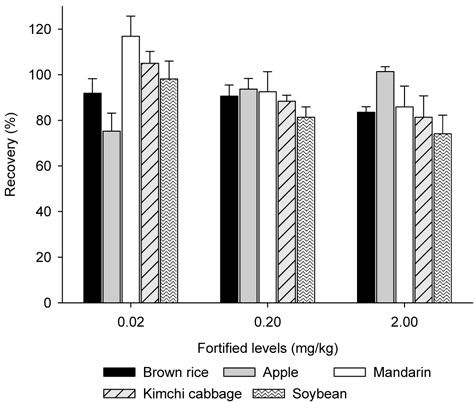
ILOQ of metazosulfuron was 2 ng (S/N ≥10) and good linearity was achieved between 0.05 and 12.5 mg/Kg of metazosulfuron standard solutions, with coefficients of determination of 0.9999. MLOQ was 0.02 mg/Kg. Good recoveries from 74.1 to 116.9% with coefficients of variation (C.V.) of less than 10% were obtained, regardless of sample type, which satisfies the criteria of Korea Food and Drug Administration (KFDA). Those results were reconfirmed with LC-MS (SIM). The method established in this study is simple, economic and efficient to be applied to most of crops as an official and general method for residue analysis of metazosulfuron.
Pesticides have been used to protect food crops from pests (diseases, insects, weeds, nematodes and etc.) for many years. Approximately one-third of the world’s food crops is destroyed by pests during growth, harvesting and storage (Ware and Whitacre, 2004).
However, with their use, the risk of residues remaining on the food is a major concern of food safety issues. Legislations were enacted through the world to regulate pesticides in food products (Ahmed, 2001). The pesticide residue levels in foodstuffs are controlled by MRLs (maximum residue limits) (Torres
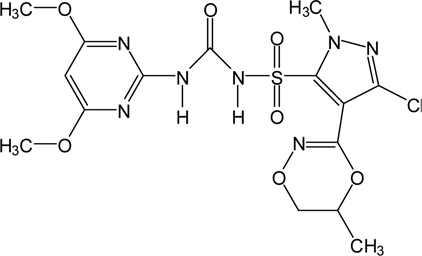
Metazosulfuron [1-{3-chloro-1-methyl-4-[(5RS)-5,6-dihydro- 5-methyl-1,4,2-dioxazin-3-yl]-pyrazol-5-ylsulfonyl}-3-(4, 6-dimethoxypyrimidin-2-yl)urea] (Fig. 1), the subject pesticide, is a pyrimidinyl sulfonylurea herbicide developed by Nissan Chemical Industries, Ltd. (Lee
Sulfonylurea herbicides interfere with acetolactate synthase (ALS), the enzyme responsible for synthesis of branched-chain amino acids such as valine, leucine, and isoleucine. The inhibition of this enzyme disrupts the plant’s ability to manufacture proteins, with such disruption leading subsequently to the cessation of all cell division and eventual death of the plant (Krämer
Analysis of sulfonylurea herbicide residues were reported for grains (Ishimitsu
The purpose of this study is to develop a novel analytical method of metazosulfuron residues in crop/food using HPLC, which can be used generally and officially for many different crop/food samples through full method validation. As crop samples for study, representative crops were selected among five crop groups such as cereal, fruits, vegetables, beans/oily crops and potatoes.
>
The subject pesticides and crops
Metazosulfuron (91.3%, purity) (Fig. 1) was obtained from Kyung Nong Corporation (Seoul, Korea). Brown rice, apple, mandarin, Kimchi cabbage and soybean of "residue-free grade" were purchased from local market. They were chopped, macerated and kept in a freezer at a temperature below -20°C in polyethylene bags.
>
Chemicals, reagents and standard solutions
Acetonitrile, acetone,
>
Measurement of instrumental sensitivity and calibration curve linearity
Metazosulfuron standard solutions (0.05, 0.1, and 1 mg/L) were analyzed with HPLC and the S/N (Signal/noise ratio) of metazosulfuron peak on chromatograms was calculated for ILOQ. The standard solutions at concentrations of 0.05, 0.1, 1, 5, 10 and 12.5 mg/L were analyzed with HPLC and the calibration curve linearity (R2) was measured.
>
Establishment of the HPLC condition for the separation of metazosulfuron in crop samples
HPLC analysis was performed using an Agilent HPLC 1100 series system (Delaware, USA) with Agilent Eclipse XDB-C18 column or YMC-Pack pro C18 (250 mm × 4.6 mm i.d., 5 μm particles) at 35°C. Mobile phase was 0.1% formic acid in acetonitrile (A) and 0.1% formic acid in water (B) (flow rate : 1 mL/min). For the analysis of brown rice and apple samples with Agilent Eclipse XDB-C18 column, 60 : 40 (v/v, A : B) was used as mobile phase, and 65 : 35 (v/v, A : B) for mandarin, Kimchi cabbage and soybean samples with YMC Pack-pro C18 column. The injection volume was 20 μL and the detection wavelength of metazosulfuron in crop samples was at 245 nm.
For the selection of optimum detection wavelength of metazosulfuron, an aliquot (20 μL) of a standard solution (10 mg/Kg) was analyzed to get the full UV spectrum with DAD (diode-array detector; 180-400 nm) under isocratic elution (acetonitrile : water = 50 : 50, v/v).
>
Clean-up with Florisil® and silica gel glass column or SPE cartridge
Glass column clean-up systems were prepared by packing of Florisil® (10 g) or silica gel (10 g) into glass column (15 × 350 mm). Those columns and two Florisil® and silica gel SPE cartridges were conditioned with
>
Clean-up with alumina N SPE cartridges
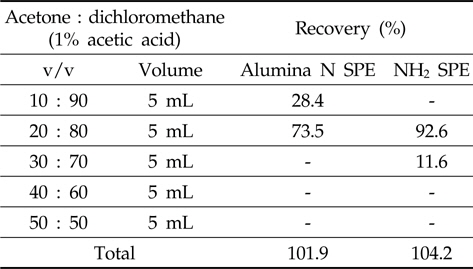
The alumina N SPE was conditioned with dichloromethane before loading the metazosulfuron standard solution (1 mL of 1 mg/L) and then metazosulfuron was eluted from SPE cartridges with several compositions of acetone or methanol/dichloromethane with 1% acetic acid (10/90, 20/80, 30/70, 40/60, and 50/50; v/v) in sequence.
>
Clean-up with NH2 SPE cartridges
The NH2 SPE was conditioned and eluted as for alumina N SPE with several compositions of acetone/dichloromethane with 1% acetic acid.
Each eluate from Florisil®, silica gel column and SPE, alumina N and NH2 SPE was concentrated under reduced vacuum and the residue was dissolved with acetonitrile and analyzed with HPLC.
>
Establishment of the partitioning condition of metazosulfuron
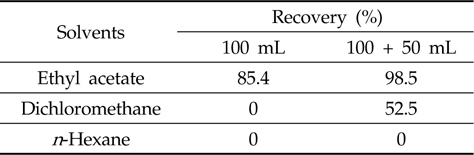
For the optimization of the liquid-liquid partitioning system, an aliquot of metazosulfuron solution (1 mL, 5 mg/L) was added to water (25 mL) and stood about 30 min in a separatory funnel before water (50 mL) and saturated sodium chloride solution (50 mL) were added. The mixture was extracted with each portion of three solvents (dichloromethane,
In the establishment of the fat removal system for such case of soybean, an aliquot of metazosulfuron solution (1 mL, 5 mg/L) was added to 50 mL of
>
Recovery test of metazosulfuron in various crop samples
Samples (25 g) of brown rice and soybean were macerated and fortified with metazosulfuron standard solution at 0.02 mg/Kg, 0.2 mg/Kg and 2 mg/Kg levels before the samples were extracted with acetonitrile (100 mL) by shaking (Reciprocal shaker SA-2s, Taitec, Japan) at 180 rpm for 1 h. The mixture was filtered under reduced pressure through a WhatmanTM GF/A filter paper and the filter cake was rinsed with acetonitrile (30 mL). The filtrate was concentrated under vacuum at 40°C. The concentrate was dissolved in ethyl acetate (100 mL), and then water (50 mL) and saturated sodium chloride solution (50 mL) were added for partitioning by shaking. Partitioning was repeated once more with 50 mL of ethyl acetate and the combined ethyl acetate layer was dried over anhydrous sodium sulfate, concentrated, and the residue was dissolved in 20% acetone/dichloromethane (5 mL). After loading the extract (1 mL) on the NH2 SPE column, which was conditioned with dichloromethane (10 mL), the cartridge was washed with 10 mL of acetone/dichloromethane (50 : 50, v/v) and eluted with 10 mL of acetone/dichloromethane (1% acetic acid) (30 : 70, v/v). The eluate was concentrated, dissolved with acetonitrile (1 mL), and analyzed with HPLC.
>
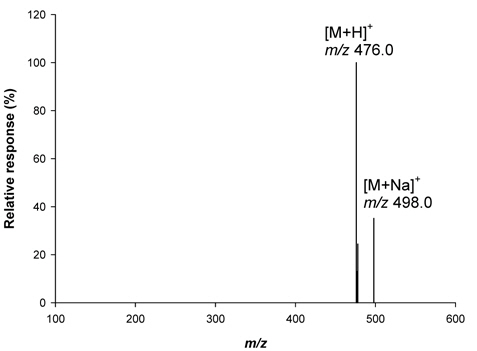
Optimization of [M+H]+ ion (m/z 476) of metazosulfuron in LC-MS and analysis of crop samples
Varian 500-MS IT-MASS spectrometer (California, USA) equipped with Shiseido Nanospace SI-2 HPLC (Tokyo, Japan) was used with Phenomenex Kinetex C18 column (100 mm × 2.1 mm i.d., 2.6 μm particles, California, USA). Elution solvent for HPLC was 0.1% formic acid in acetonitrile and 0.1% formic acid in water (50 : 50, v/v) (flow rate : 0.2 mL/min). Drying gas temperature, drying gas pressure, and nebulizer gas pressure were 350°C, 40 psi and 30 psi, respectively.
A standard solution (1 mg/Kg) of metazosulfuron (5 μL) was analyzed using LC-MS in ESI (Electrospray ionization) positive mode (mass range :
Although the MRL of metazosulfuron was established only for rice (0.05 mg/Kg), apple and mandarin from fruits, Kimchi cabbage from vegetables, potato from potatoes, and soybean from beans and oil crops were selected as representative crops, in consideration of their popularity and matrix characteristics.
>
HPLC conditions for analysis of metazosulfuron
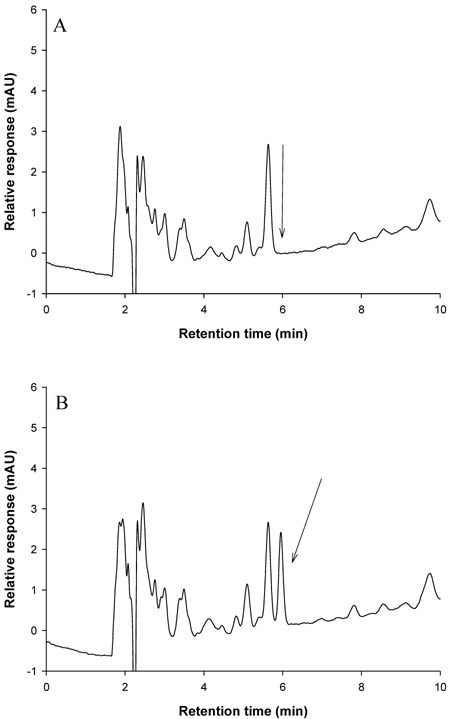
Optimum detection wavelength of metazosulfuron in HPLC was investigated for sensitive detection. When a full UV spectrum of metazosulfuron was recorded using DAD, λmax was observed at 245 nm, which was used as a detection wavelength in this study. Other sulfonylurea herbicides also engaged 245 nm as a detection wavelength for azimsulfuron, flazasulfuron, and halosulfuron-methyl (Ishimitsu
>
Establishment of clean-up procedure with glass column and SPE cartridge
In pesticide residue analysis adsorption chromatography is generally used for the clean-up of the interfering coextractives (e.g. lipids and pigments) which were not removed by liquid-liquid partitioning. It depends on the existence of weaker van der Waals forces and/or hydrogen bonding (Fong
In the first trial with Florisil®/silica gel glass column and Florisil®/silica gel SPE cartridges, the recoveries of metazosulfuron were very low (37-53%, data not shown) when eluted with various combinations of acetone/
[Table 1.] Recovery rate by sequential elution of acetone/dichloromethane with 1% acetic acid
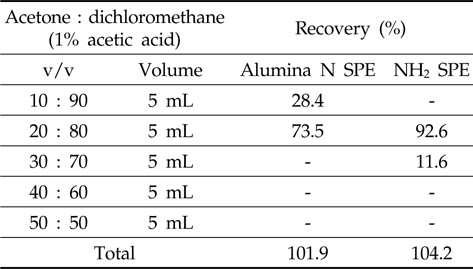
Recovery rate by sequential elution of acetone/dichloromethane with 1% acetic acid
>
Liquid-liquid partitioning of metazosulfuron
After the successful establishment of the clean-up procedure, the liquid-liquid partitioning system was examined. Liquid-liquid partitioning of sample extract between immiscible solvents, such as water versus organic solvents, removes the polar interfering coextactives (e.g. carbohydrates) (Fong
[Table 2.] Efficiency of liquid-liquid partitioning with three different solvents
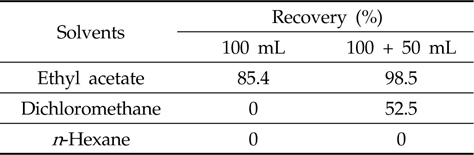
Efficiency of liquid-liquid partitioning with three different solvents
Method validation is a set of procedures to evaluate the performance characteristics such as recovery, linearity and range of calibration, limits of detection and quantitation of a method for specific analyte and sample types (Codex Alimentarius Commission, 2003).
ILOQ (Fong
Method Limit of Quantitation (MLOQ) is a practical LOQ of the total analytical method. It is usually calculated using ILOQ, injection volume, final extract volume and sample weight in the analytical method (Equation 1) (Lee
MLOQ (mg/Kg) = (2 ng × 5 mL) / (20 μL × 25 g) = 0.02 mg/Kg___________Equation 1
MLOQ value for metazosulfuron was calculated using equation 1 was 0.02 mg/Kg. This value satisfied the criteria of KFDA which is below 0.05 mg/Kg or half of MRL (Lee, 2012).
The selectivity of the analytical method was reasonable because there were no interfering peaks at the retention times of metazosulfuron (5.966 min for brown rice and apple, and 6.661 min for mandarin, Kimchi cabbage, and soybean).
Recovery test can provide accuracy and precision of the sample preparation method by the recovery rate (accuracy, %) and coefficients of variation (C.V.) (precision, %) (Fong
Comparing with the reported analytical methods with other sulfonylurea herbicides (Ishimitsu
>
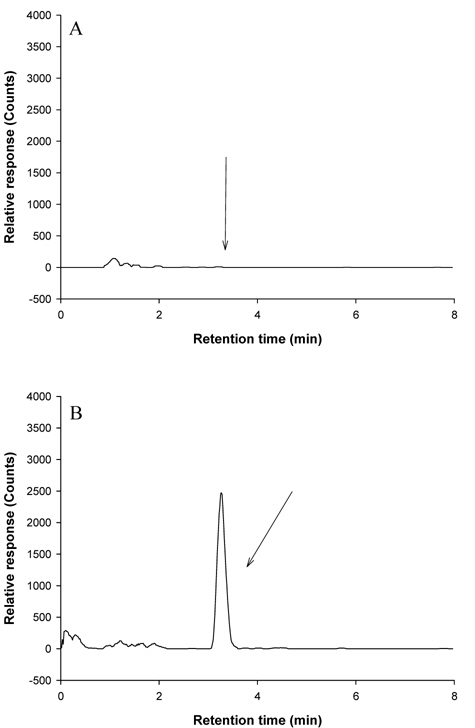
Confirmation of metazosulfuron in crop matrices by LC-MS(SIM)
LC-MS was used for the confirmation of the recovered metazosulfuron residues in crop samples. Before sample analysis, three parameters (capillary voltage, RF loading, and needle voltage) of LC-MS were tuned to get the highest sensitivity of metazosulfuron. Capillary voltage affects the focusing of ions into the capillary as well as the energy imparted to them as they exit the capillary and enter the skimmer. RF Loading (%) optimizes the amount of energy that an ion acquires after injection into the ion trap. The needle voltage is the voltage applied to the tip of the spray needle to make charged fine droplets with the aid of a nebulizer gas. By using a combinationthese three, the full scan spectrum of metazosulfuron gave