



Recent treatment regimens of some chronic rheumatic diseases (RD) comprise multimodal concepts including pharmacologic, physical/exercise, occupational and psychological therapies to support the long-term management of disease. Spa therapy is widely used for musculoskeletal system disorders, such as low back pain, Fibromyalgia or Osteoarthritis (OA) (Annegret and Thomas, 2013). Thermal therapy is very popular in many European and Middle Eastern countries, as well as in Japan and Israel. This treatment comprises a broad spectrum of therapeutic modalities including hydrotherapy, balneotherapy, physiotherapy, mud-pack therapy and exercise. The terms hydrotherapy and balneotherapy were accepted for all forms of treatment with water. Balneotherapy uses natural thermal mineral waters, whose definition is based on the sum of the cations Na, K and Mg and the anions SO4, Cl and HCO3; the water is generally used at a temperature of around 34 degrees Celsius. Mud-pack is defined as a natural product that consists of a mixture of mineral or mineral-medicine water with organic or inorganic material produced from biological and/or geological processes and used as a therapeutic treatment in the form of a mud wrap applied locally or to the whole body (Bender et al., 2005; Nicholas, 1999; Sukenik et al., 1999). Nowadays it represents an useful alternative to the pharmacological therapy, because of problems related to the use of drugs that often have significant side effects and the occasional lack of valid therapeutic strategies (Bresalier et al., 2005; Kearney et al., 2006; Ofman et al., 2002; Zhang et al., 2004). The efficacy of spa treatments in RD has been bolstered by ancient tradition, but in spite of their long history and popularity, only a few randomized, controlled trials (RCTs) on the use of these therapies in patients with RD have been conducted and this topic is still the subject of debate and its role in modern medicine continues to be unclear (Verhagen et al., 2007). Existing clinical researches are not sufficiently strong to draw firm conclusions; additional large and welldesigned RCTs are needed to confirm preliminary evidences.
The aim of spa therapy is to reduce pain, relieve muscle spasms and improve muscle strength and functional mobility (Bender et al., 2005; Sukenik et al., 1999). The action mechanisms of mud packs and thermal baths are not completely known, and it is difficult to distinguish the effects of thermal applications from the benefits that could be derived from a stay in a spa environment (Sukenik et al., 1999).
Although the results of the existing studies are not strong enough to draw firm conclusions, it is necessary to ask what real medical and scientific value these therapies have. The objective of this review is to summarize the currently available information on mechanisms of action and possible effects of spa therapy in RD. We also provide some suggestions for further development in this area.
>
Mechanisms of action of spa therapy in RD
The mechanisms by which immersion in thermal mineral water alleviates suffering in RD are not fully understood. The net benefit is probably the result of a combination of factors, among which the mechanical, thermal and chemical effects are most prominent (Sukenik et al., 1999).
A distinction can be made between the non-specific (hydrotherapeutic in a broad sense) mechanisms common to simple baths in hot tap water, and specific (hydromineral and crenotherapeutic) mechanisms, which depend on the chemical and physical properties of the water used. While the former are well known, the latter are difficult to identify and assess (Sukenik et al., 1999).
>
Mechanical, thermal and chemical effects
Spa therapy may have beneficial effects on muscle tone, joint mobility, and pain intensity.
Increased buoyancy and hydrostatic pressure during immersion in thermal mineral water cause many physiologic changes. Immersion to the suprasternal notch in mineral water (35℃) results in a cascade of reactions including increased diuresis, natriuresis, and cardiac output (Epstein, 1992; O’Hare et al., 1985; Weston et al., 1987). The basis of these physiological effects is considered to be the hydrostatic pressure, which forces approximately 700 ml from the lower extremities to the central compartment. Distension of the volume receptors by this central hypervolemia is regarded as the trigger for the observed physiological effects (Epstein, 1992; O’Hare et al., 1985; Weston et al., 1987).
The effects of thermal baths are partially related to temperature. Hot stimuli may influence muscle tone and pain intensity, helping to reduce muscle spasm and to increase the pain threshold in nerve endings. According to the “gate theory”, pain relief may be due to the temperature and hydrostatic pressure of water on the skin (Melzack and Wall, 1965).
Thermal stress provokes a series of neuroendocrine reactions. In particular, the heat stimulates the release of adrenocorticotropic hormone (ACTH), cortisol, prolactin and growth hormone (GH), although it does not alter the circadian rhythm of these hormones (Kuczera and Kokot, 1996).
The effect of thermal stress on the hypothalamus-pituitary-adrenal axis seems to be particularly important for the antiedemigenous and anti-inflammatory actions of corticosteroids, as well as for the frequent alteration of the axis during some RD (Gur et al., 2004). The increase in betaendorphin demonstrated to occur with various spa therapy techniques has an analgesic and anti-spastic effect that is particularly important in patients for whom pain is the prevalent symptom (Cozzi et al., 1995; Kubota et al., 1992).
Recent data have demonstrated the possibility that normal keratinocytes can produce and secrete a precursor pro-opiomelanocortin (POMC) following various stimuli (e.g. ultraviolet rays, thermal stimuli) which is the common precursor of various endorphins (Ghersetich et al., 2000). This finding allows us to formulate the fascinating hypothesis that ultraviolet radiation or thermal stimuli could be used to condition the skin’s production of opioid peptides, thus altering the personal emotional sphere or pain threshold. If we add that ß-endorphin also has immunomodulatory effects, the hypothesis of a close correlation between spa therapy and the psychoneuroendocrine system becomes increasingly convincing (Berczi et al., 1996).
Furthermore, hyperthermia plays an important role on immune system function (see below).
Hyperthermia also has many effects on granulocytes. Heat increases their mobility, phagocytic and bactericidal properties and enzymatic activity (Sukenik et al., 1999).
Furthermore, thermal stimulation increases the extensibility of collagen-rich tissues, such as tendons, fasciae and articular capsules, which may improve the range of motion of joints (Sukenik et al., 1999).
The effects described make it possible to break the vicious circle of pain-muscle contraction-altered joint dynamics-pain that characterizes many chronic arthropathies. The reduction of muscle tone and better use of joints represent just two of the most important elements that show the medium and long-term beneficial effects documented in various clinical studies (Cantarini et al., 2007; Elkayam et al., 2000; Fioravanti et al., 2007; Fioravanti et al., 2010; Forestier et al., 2009; Guillemin et al., 1994; Harzy et al., 2009; Nguyen et al., 1997; van Tubergen et al., 2001).
The chemical effects of mud-packs and thermal therapy are less clear than the physical effects. In theory, it cannot be excluded that the organic substances or minerals of water or mud, sometimes present in traces, can be absorbed through the skin and then act at a systemic level. However, experimental evidence available in this field is scarce. Shani et al. (1985) documented a significant increase in serum concentrations of bromine, rubidium, calcium and zinc in patients with psoriatic arthritis who bathed in the Dead Sea. The penetration of the solutes is presumably influenced by the length of bathing time, the temperature of the thermal water, its composition and other factors, some of which may still be unknown. Furthermore, it has been reported that the direct application of mud-pack has greater clinical effects than the application of nylon covered mud pack in patients with knee OA (Odabasi et al., 2008).
Since sulphur baths have been successfully used in various skin immunomediated afflictions, it has been suggested that absorption through the skin of trace elements present in mineral water and mud packs may affect the immune system (Sukenik et al., 1997).
Overall, thermal stress has an immunosuppressive effect. With regard to hyperthermia a stimulatory effect of the immune response appear to prevail at a moderate increase of local skin temperature, with increase of pro-inflammatory cytokines interleukin (IL)-6 (Sobieska et al., 1993) and IL-1β (Olszewski et al., 1989), whereas higher temperatures (40 - 41℃) apparently suppress immune functions (Lange et al., 2006; Schmidt and Simon, 2001).
A significant reduction in circulating levels of T-lymphocytes has been demonstrated in healthy volunteers treated with hyperthermal baths (Sukenik et al., 1999) and in patients with respiratory and cutaneous atopy (Valitutti et al., 1990). Hyperthermia-induced T-lymphocytopenia and eosino penia may be due to a redistribution of the cells, probably due to the increase of ACTH and cortisol provoked by thermal stress (Kuczera and Kokot, 1996).
In vitro studies have demonstrated that sulphurous waters have a dose-dependent inhibitory effect on the blast transformation and proliferation of T lymphocytes obtained from peripheral blood in both healthy subjects and subjects affected by chronic inflammatory diseases (Valitutti et al., 1990). On the other hand, immersion in thermal waters at a temperature of 40℃ reduces the lymphocyte response to phytohaemoagglutinin (Smith et al., 1978). Sulphurous waters also seem to exert a potent inhibitory action on the production of cytokines, especially IL-2 and interferon gamma (IFN-γ). As these cytokines are mainly produced by CD4+ lymphocytes, it can be hypothesized that memory T cells are the principal target of sulphur-rich waters. The application of sulphurous waters reduces the capacity of memory T cells to proliferate and produce cytokines, thus resulting in an alteration of immune response (Ghersetic and Lotti, 1996). The hyperthermia-induced alterations of the cytokine milieu has been recently confirmed in patients affected by ankylosing spondylitis (AS) (Tarner et al., 2009). Tarner et al. (2009) showed that the serum levels of tumor necrosis factor (TNF)-α, IL-1β and IL-6 which were measured before, during and after whole-body hyperthermia, were significantly reduced in patients with AS whereas the changes in healthy subjects did not reach statistical significance.
>
Anti-inflammatory and chondroprotective aspects
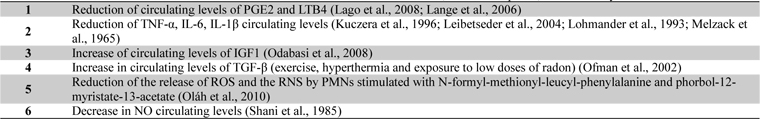
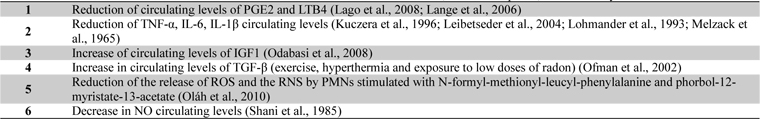
Recent studies have shown a reduction of circulating levels of Prostaglandin E2 (PGE2) and Leukotriene B4 (LTB4), important mediators of inflammation and pain, in patients suffering from OA or fibromyalgia who undergo mud-packs or balneotherapy (Ardiç et al., 2007; Bellometti and Galzigna, 1998) (Table 1).
Effect of thermal mineral baths on various mediators or factor of inflammation, immune response, and chondrolysis
Crenotherapy also affects the synthesis of various cytokines involved in the ongoing chondrolysis and inflammation in RD; in fact a reduction in the cytokines IL-1β and TNF-α and the soluble receptors of the latter has been demonstrated following a cycle of mud-baths therapy (temperature > 41C°) in patients with OA (Bellometti et al., 1997; Bellometti et al., 2002; Cecchettin et al., 1995) (Table 1).
Several studies have provided evidence for a significant role of matrix metalloproteinases (MMPs), particularly MMP-3 or stromelysin-1, produced by activated chondrocytes and other cell types in the development of cartilage degradation in joint diseases (Lohmander et al., 1993). A recent study of Bellometti et al. shown that MMP-3 serum levels were significantly reduced by mud-bath therapy in patient with OA (Bellometti et al., 2005).
Cycles of mud applications and balneotherapy also bring about an increase in some growth factors, such as Insulin-like Growth Factor 1 (IGF1) (Bellometti et al., 1997), which stimulates cartilage anabolism (Trippel, 1995). Furthermore, a significant increase in circulating levels of Transforming Growth Factor-beta (TGF-β) has been found in patients with AS after a combined spa-exercise therapy (exercise, hyperthermia and exposure to low doses of radon) (Shehata et al., 2006) (Table 1). TGF-β is a very potent immunomodulating and anti-inflammatory cytokine which plays a major role in tissue healing, bone remodelling and fibrosis (Shehata et al., 2006).
Among the various factors responsible for inflammatory and degenerative phenomena in joint during different RD, reactive oxygen species (ROS) and nitric oxide (NO) should be taken into consideration (Farrell et al., 1992).
Sulphurous waters have been demonstrated to have an antioxidant effect in vitro; in fact the incubation in sulphurous mineral water significantly reduces the release of ROS and the reactive nitrogen species (RNS) peroxynitrite by polymorphonucleate leukocytes (PMNs) stimulated with N-formyl-methionyl-leucyl-phenylalanine and phorbol-12-myristate-13-acetate (Braga et al., 2008). Various studies in humans have highlighted the positive action of mud-packs and thermal baths, especially sulphurous ones, on the oxidant/antioxidant system. Grabski et al. (2004) reported the reduction of superoxide dismutase (SOD) activity in patients with rheumatoid arthritis undergoing treatment with sulphuric water. Eckmekcioglu et al. (2002) demonstrated that 3 weeks of sulphur baths can reduce the antioxidative defence system (SOD and glutathione (GSH) peroxidase) in the blood of patients with OA. They discussed that the decline of these enzyme- activities may be caused by two reasons: either as consequence of reduced oxidative stress during sulphur therapy leading to a lower expression of these enzymes or as an enhanced generation of superoxide radicals exhausting the superoxide scavenging enzyme.
Bender et al. demonstrated that therapeutic baths in mineral water reduced the activity of catalase, SOD, malondialdehyde and GSH peroxidise (Bender et al., 2007). Other authors have observed a significant decrease in NO and myeloperoxidase and a slight increase in GSH peroxidase in the sera of subjects with OA undergoing cycles of mud applications and balneotherapy (Bellometti et al., 2000).
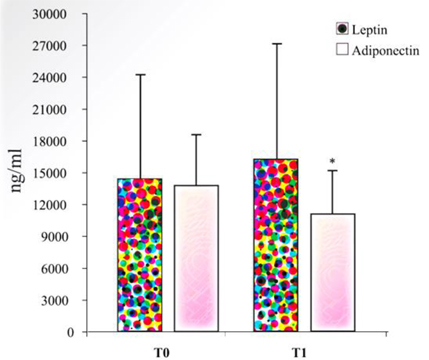
In a recent study we assessed the possible modifications of plasma levels of leptin and adiponectin in patients with OA treated with a cycle of mud-bath therapy (Fioravanti et al, 2011). Our data showed at the end of the therapy, a slight but not significant increase of plasma leptin concentrations and a significant decrease in serum adiponectin levels (Fig. 1). These adipocytokines play an important role in the pathophysiology of OA (Lago et al., 2008). In particular, there is some evidence that adiponectin in skeletal joints may have proinflammatory effects and may be involved in cartilage degradation (Gomez et al., 2009). In view of these recent findings, the decrease of adiponectin after spa therapy demonstrated in our study may play a protective role in OA.
Partial contradictory data were observed in a recent pilot study conducted by Shimodozono et al. (2012). Authors reported a significant increase of serum levels of leptin in 7 healthy men after a single 10 min warm-water bath (WWB) at 41℃ with tap water or WWB with inorganic salts and carbon dioxide (ISCO2), and remained significantly higher than those at baseline even 30 min after WWB with tap water; serum levels of adiponectin showed a slight, but not significant, increase immediately and 30 min after a single WWB under both conditions. These differences might be due to the difference in subjects (relatively healthy, young, lean and male in this last study) and in modalities of bathing (duration of thermal stimulation and the substances used in the mineral water).
Experimental studies in animal models of arthritis corroborate the evidence of beneficial effects of mud-bath therapy on inflammatory and degenerative joint diseases. Cozzi et al. (Cozzi et al., 2004) have recently demonstrated an antiinflammatory effect of mud–bath applications in Freund’s adjuvant-induced arthritis in rats. In 2007 Britschka et al. (2007) confirmed the anti-inflammatory and chondroprotective effects of the application of mud in Zymosan-induced arthritis in rats, by performing histological analysis on synovial tissues and cartilage taken from the sacrificed animals on day 21 of treatment.
The possible chondroprotective role of mineral water or mineral components was confirmed by some pilot
A recent study by Fioravanti et al. (Fioravanti et al., 2013) showed similar results in human OA chondrocytes cultivated with mineral Vetriolo’s thermal water (VW) in the presence or in the absence of IL-1β. OA chondrocytes were cultivated in Deionized Water (DW) (DW-DMEM, controls), or in one of three different VW-DMEM media, in which DW had been totally (100%) or in part (50% or 25%) substituted with VW. The results showed that VW alone at 25% or 50% concentration did not affect the viability of cultured OA chondrocytes, and determined a significant survival recovery rate in cultures stimulated with IL-1β. On the contrary, the VW alone at 100% of concentration reduced, in a significant (
Many other non-specific factors may also contribute to the beneficial effects observed after spa therapy in some RD, including effects on cardiovascular risk factors.
The lipid normalizing effects of balneotherapy, especially with sulphurous waters, have been reported for decades. The results of such research have documented reductions in total cholesterol, triglycerides and nonesterified cholesterol and a significant increase in HDL- cholesterol (Strauss-Blasche et al., 2003).
More recently, attention has focused on plasma homocysteine, a risk factor for coronary heart disease, congestive heart failure, systolic hypertension, artherothrombotic events, complications in diabetes mellitus, cancer and oxidative stress (Agullo-Ortuno et al., 2002; Bostom and Selhub, 1999; Sun et al., 2002; Vasan et al., 2003). A significant reduction in plasma homocysteine has been demonstrated in OA patients after a cycle of sulphurous thermal baths (Leibetseder et al., 2004).
Recently Oláh et al. (Oláh et al., 2010) explored changes in several cardiovascular risk factors in a group of patients suffering from degenerative musculoskeletal disorders subjected to a cycle of balneotherapy. For the first one, the Authors showed a statistically significant and lasting (3 months after the cycle of balneotherapy) decrease in serum levels of high- sensitivity C-reactive protein (hs-CRP) in patients treated with mineral thermal baths.
Always Oláh et al. (Oláh et al., 2011) in a consecutive study explored the changes of antioxidant, inflammatory and metabolic parameters in obese and hypertension people after balneotherapy. This study showed that balneotherapy is a safe therapeutic option in hypertension and obesity and that it improved some metabolic and inflammatory markers, such as hs-CRP on haemoglobin A1C levels.
The reduction of cardiovascular risk factors through mudpacks and spa therapy is especially important considering the clear and much-stressed association between various RD and atherosclerotic processes (Turesson et al., 2008).
Finally, other elements need to be taken into consideration concerning the mechanisms of action of mud applications and spa therapy in RD, such as the particular climatic and environmental conditions of spas. One of the most important aspect of spa therapy is the psychologic effect of removal from the stress of home and work. Those receiving spa therapy experienced not only reduced pain and improved function but also experienced greater physical and mental quality of life and less anxiety and depression (Bender et al., 2005; Sukenik et al., 1999).
In this review we highlighted the effects of spa therapy and mud applications on various mediators or factors of inflammation, immune response and chondrolysis. Although the data presented are stimulating, it is impossible to ignore the existence of a complex series of problems and uncertainties that prevent spa therapies from gaining the full consensus of the scientific community (Verhagen et al., 2007). One of the critical points is the controversial problem of the absorption of the minerals dissolved in thermal waters, e.g. the demonstration of specific effects other than those linked to the simple action of heat. Unfortunately few studies have been conducted on this topic and little is known about the specific effects of various mineral waters. It is still not clear which elements are essential and what is the ideal concentration of each element in order to attain an optimal response to treatment. It remains to be clarified which mineral waters are most suitable for various diseases and whether the different components exert specific actions. Such evidence would lead to a specialization of spa resorts, which could finally target their therapies more accurately and rationally.
Furthermore, the results reported only refer to short term modifications of various mediators of inflammation, immune response and chondrolysis, lasting until the end of the cycle, and little is known of the possible long term effects. This is a key element in seeking to explain the persistence of the symptomatic benefit induced by such therapies in some RD, as shown in long term controlled clinical trials (Cantarini et al., 2007; Elkayam et al., 2000; Fioravanti et al., 2007; Fioravanti et al., 2010; Forestier et al., 2009; Guillemin et al., 1994; Harzy et al., 2009; Nguyen et al., 1997; van Tubergen et al., 2001).
In conclusion, existing researches are stimulating, but are not sufficiently strong to draw firm conclusions. More studies are needed to help draw firm conclusions regarding the mechanisms of actions of spa therapy in various RD.



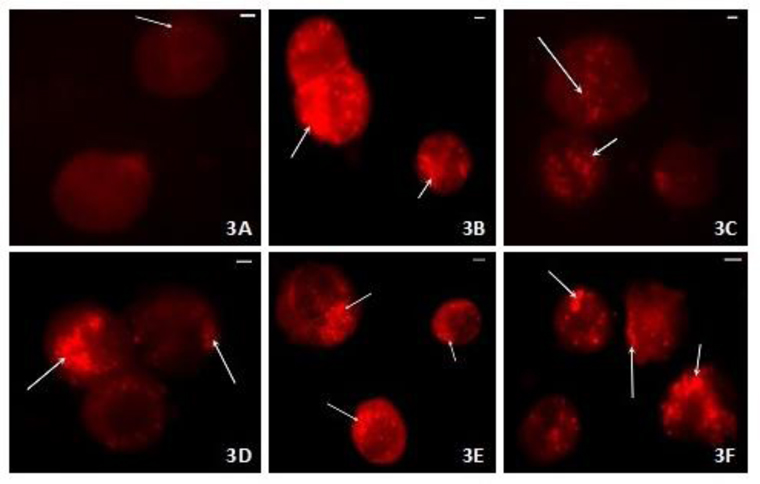
![TEM micrographs of human OA chondrocytes: (A) DW-DMEM, controls: the cell shows a good state of health. (B) Incubation with IL-1β (5 ng/ml): Vacuolization (V) of the cytoplasm and reduction in quality of Golgi bodies, rough endoplasmic reticulum (RER) and mitochondria (M). (C) 25% or 50% VW-DMEM: not particular morphological alterations. (D) 100% VW-DMEM: Altered status of chromatin and of cytoplasm structure. Normal plasma membrane and nucleus (N). (E) 25% or 50% VW-DMEM with IL-1β: The cell partially restores its morphology. Euchromatic nucleus (N), restored organization of cytoplasm [vacuoles (V) very reduced, abundant rough endoplasmic reticulum (RER), well shaped mitochondria (M)]. Plasma membrane with cytoplasmic processes. (F) 100% VW-DMEM with IL-1β: Vacuoles (V), reduction of cytoplasmic organelles, and nucleus (N). Bar = 1 μm (from Fioravanti et al., 2013).](http://oak.go.kr/repository/journal/15272/TJHOBI_2014_v4n1_31_f003.jpg)