Cinnamomum zeylanicum (C. zeylanicum) is a tropical evergreen tree of Lauraceae family. It is one of the oldest culinary spices known and used traditionally in many cultures for centuries. In addition to its culinary uses, cinnamon also possesses as a folk remedy of many health disease condition including analgesic, antiseptic, antispasmodic, aphrodisiac, astringent, carminative, haemostatic, insecticidal, and parasiticide and memory enhancing property. This study was aimed to assess the acetylcholinesterase and butyrylcholinesterase inhibitory activity of standardized methanol extract of the C. zeylanicum. Gas chromatography - mass spectrometry (GC-MS) and high performance liquid chromatography (HPLC) analysis were done to identify the presence of eugenol as chemical component and support the neuroprotective activity in the extract. Anticholinesterase inhibitory activity of crude methanol extract of C. zeylanicum leaves and cinnamon oil were evaluated by 96-well microtiter plate assay and thin layer chromatography bioassay detection methods. This study revealed that cinnamon oil (IC50: 45.88 ± 1.94 μg/ml) has better anticholinesterase activity than methanol extract (IC50: 77.78 ± 0.03 μg/ml). In HPLC analysis, retention time of eugenol in cinnamon oil was found to be 15.81 min which was comparable with the retention time (15.99 min) of the reference standard, eugenol. Seven chemical compounds were identified by GC-MS analysis, in which eugenol as an important phytoconstituents. Thus the phytochemicals from C. zeylanicum methanol leaves extract could be developed as potential source of anticholinesterase activity, with particular benefit in the symptomatic treatment of Alzheimer’s disease.
Alzheimer’s disease (AD) is a progressive and complex neurodegenerative disorder of the brain. Pathophysiology of the disease suggests that deposition of amyloid-β, accumulation of ᶩ-protein, release of inflammatory mediators and decreasing label of acetylcholine in the cholinergic system could be the possible reason for enhancing neurodegenerative condition of the AD brain (Mukherjee et al., 2007b). It is well known that patients with AD were deficient of acetylcholine, neuro transmitter that navigates the memory process of central nervous system. This fact has led to develop the cholinergic hypothesis, which focuses impairment of cognitive function in AD related disorder. The specific events of AD caused due to termination of the neurologic impulses at cholinergic synapses (White et al., 1977). It escalates to chemist to build a suitable cholinesterase inhibitor as potential model compounds for the treatment of several central nervous system disorders including AD. Synthetic cholinesterase inhibitors such as tacrine, donepezil, rivastigmine and natural origin galantamine have been approved for symptomatic treatment for the mild to moderate form of the AD. These inhibitors are primarily nature of competitive or non-competitive type for true acetyl cholinesterase (AChE) and pseudo butyryl cholinesterase (BChE) or for both isoforms. Searching for alternatives, chemical entity of natural origin and its derivatives have been tested scientifically and employed successfully in the treatment of cognitive disorders (Mukherjee and Houghton, 2009; Mukherjee et al., 2007a).
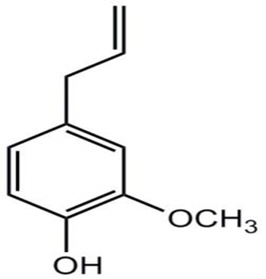
Acetylthiocholine iodide (ATCI), Butyrylthiocholine iodide (BTCI), Ellman’s reagent (5, 5’-dithiobis-(2-nitrobenzoic acid) (DTNB), AChE from bovine erythrocytes, BChE from equine serum, galantamine and standard eugenol was procured from Sigma (Poole, UK). Methanol and all other organic solvents (HPLC reagent grade) were purchased from Merck, India.
Shade dried powdered leaves (120 g) of
To obtain the cinnamon oil from leaves of
In this method, 2.5 mm silica gel TLC plate F254 (Merck, Darmstadt, Germany) plates were pre-saturated with an appropriate solvent and thoroughly dried just before use. The
96-well microtiter plate method for detection of AChE and BChE inhibition
Cholinesterase is the principal enzyme of central nervous system which hydrolyzes the substrate acetylthiocholine/ butyrylthiocholine into prime end product thiocholine which reacts with Ellman’s reagent (DTNB) to produce 2-nitrobenzoate-5-mercaptothiocholine and 5-thio-2-nitroben zoate which appears as yellow in color and detected at wavelength of 405 nm. AChE/BChE inhibitory activity of methanol extract, cinnamon oil, eugenol and galantamine were studied in 96-well microtiter plate (Bio Rad, 680 XR, USA) method (Bhadra et al., 2011; Ellman et al., 1961). Galantamine was used as the standard cholinesterase inhibitor. 125 μl of 3 mM DTNB, 25 μl of 15 mM ATCI (BTCI when measured the BChE inhibition), 50 μl of buffer and sample was dissolved in phosphate buffer and added in increasing order to each well of 96-well plate and the absorbance was read at 405 nm every 13 s for 65 s. 25 μl of 0.22 U/ml of AChE or BChE was added in each well and the absorbance was measured at every 13 s for 104 s. Each concentration was analyzed for six times. The % inhibition of the enzyme activity of each test compound concentration was calculated by plotting graph between logarithm of inhibitor concentration in the assay solution and % enzyme inhibition. The concentration of enzyme inhibitor was expressed as milligram of test substance per milliliter. The linear regression parameters were determined for each curve and the IC50 values of each test compound were calculated by using range of concentration.
>
Quantification of eugenol in the cinnamon oil by HPLC analysis
The quantification of eugenol in the cinnamon oil was carried out by Reverse-phase (RP)-HPLC (Shimadzu Prominence, Kyoto, Japan). It is equipped with two Shimadzu LC-20 AD UFLC reciprocating pumps, a variable Shimadzu SPD-M20A Prominence PDA detector and a Rheodyne manual injector with a loop size of 20 μl. Analytical samples were filtered through 0.45 μm ultra membrane filters (Millipore, Germany) before injection. C18 column (Phenomenex-Luna C18, Torrance, CA, USA) (250 × 4.6 nm, 5 μm particle size) was used as stationary phase. Isocratic elution was performed using the mobile phase methanol: 1% acetic acid in deionized water (90 : 10, v/v), at a flow rate of 1 ml/min. Detection of the compounds were performed at 254 nm. LC solution software was used to calculate the peak area of standard curve. Identification of standard peak was achieved by comparison of the retention time (Rt) of cinnamon oil with standard. The amount of standard eugenol present in the extract was estimated from calibration curve.
>
Qualitative analysis of eugenol through GC-MS
The identification and quantification of eugenol present in cinnamon oil was performed by gas chromatography coupled to mass spectrometry (GC/MS - Shimadzu QP Model 5050A) under the following experimental conditions: fused silica capillary column (30 m × 0.25 mm) DB5 bonded phase (0.25 mm thick film); Helium as carrier gas at a flow rate of 1.0 ml per min, keeping the temperature was programmed 50℃ for 1.5 min, followed by an increase of 4℃ per min up to 200°C, then at 10℃ until reaching 250°C were kept constant at this temperature for 5 min; injector temperature: 250℃ and detector temperature (or interface) 280℃. Injection volume 0.5 μl was used with split ratio of 1 : 25. Cinnamon oil diluted in methanol at a concentration of 0.1 mg/ml was used for experimental study. The retention indices of the eugenol were determined with relative value of
Results of the data were expressed as mean ± SEM. Dose response curve was performed in graph pad prism software version 4.01 (La Jolla, USA). IC50 value of inhibitors was calculated between results of percentage of inhibition versus the concentrations of sample.
In this work, hydro-distillation process was successfully applied to extract the cinnamon oil from cinnamon leaves. It was transparent, yellow colored with smell of spice. % yield of the oil was found to be 3.4% (v/w).
TLC bio-autographic assay was carried out to evaluate the cholinesterase inhibitory activity of standardised plant extract. It was found that methanol extracts of
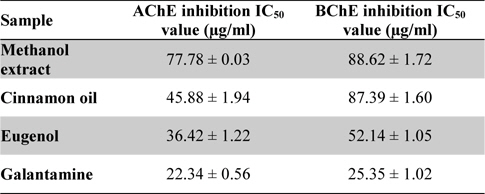
The result of 96-well microtiter plate methods for methanol extract, cinnamon oil, eugenol and standard galantamine has been shown in Table 1. The data value indicates the test and standard chemical compounds have the potential cholinesterase inhibitory activity. Cinnamon oil has shown potent AChE inhibition with IC50 value of 45.88 ± 1.94 μg/ml than methanol extract having IC50 value of 77.78 ± 0.03 μg/ml. However, AChE activities of cinnamon oil showed least activity than the standard eugenol compound (IC50: 36.42 ± 1.22 μg/ml) used. Moreover, the reference compound galantamine have shown the stronger AChE activity (IC50: 22.34 ± 0.56 μg/ml) than the standard eugenol, cinnamon oil and methanol extract. In contrast to BChE activity, the cinnamon oil (IC50: 87.39 ± 1.60 μg/ml) have shown the slight potent enzyme activity than the methanol extract (IC50: 88.62 ± 1.72 μg/ml) of
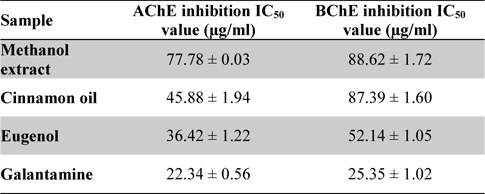
IC50 values of the methanol extract, cinnamon oil, eugenol and galantamine against acetylcholinesterase and butyrylcholinesterase
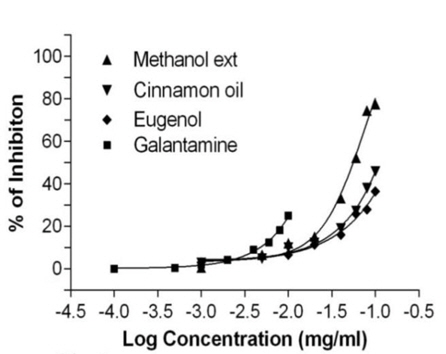
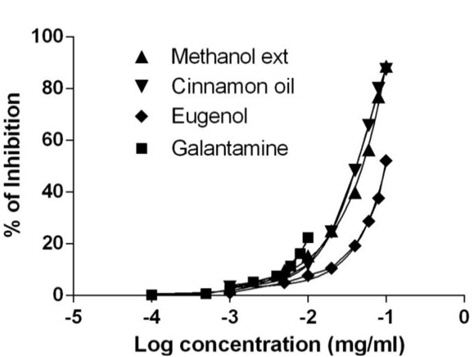
Similarly, the standard biomarker compound eugenol have shown potent BChE activity with IC50 value of 52.14 ± 1.05 μg/ml and found to be active than the cinnamon oil and methanol extract. The reference compound galantamine have shown IC50 value 25.35 ± 1.02 μg/ml and possess as most active against BChE. Data value (Table 1) was also determining the cholinesterase enzyme affinity of the studied substances towards more AChE than BChE. Dose response curve (Figs. 2- 3) of the methanol extract, cinnamon oil, eugenol and galantamine was shown in increase in percentage of AChE and BChE inhibition with increase in concentration of test samples. The cinnamon oil from
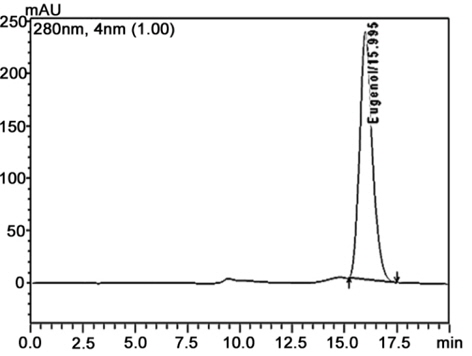
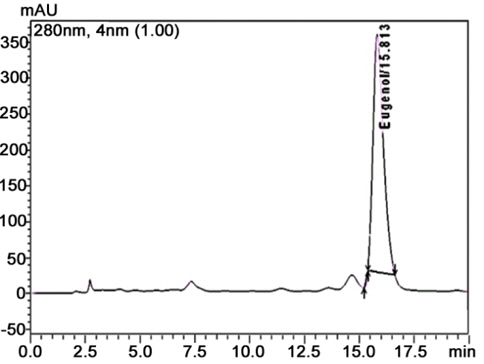
RP-HPLC method was carried out to know the purity of natural product extract (Ross, 1976). The complex mixture of the chemical compound in natural product was separated on the basis of the affinity towards stationary phase and mobile phase gradient. The label of eugenol in cinnamon oil was calculated from linear regression equation (y = 1.519065e + 007X + 1659936). The following equation showed a good agreement between peak area and used concentration (0.1 - 1.0 μg/ml) with a correlation coefficient, r2 = 0.984. The identity of eugenol in cinnamon oil was affirmed by comparing the retention times of reference compound. Chromatogram of HPLC analysis showed retention time of eugenol in cinnamon oil was 15.81 min (Fig. 4a and b). The percentage content of eugenol in cinnamon oil was found to be 0.205 mg per ml.
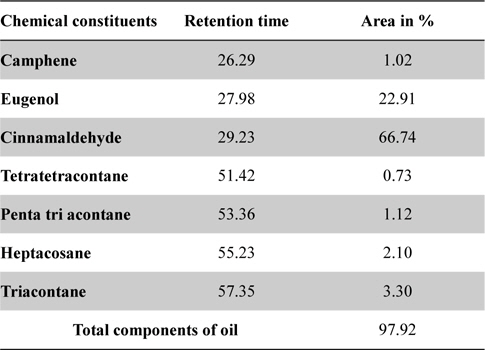
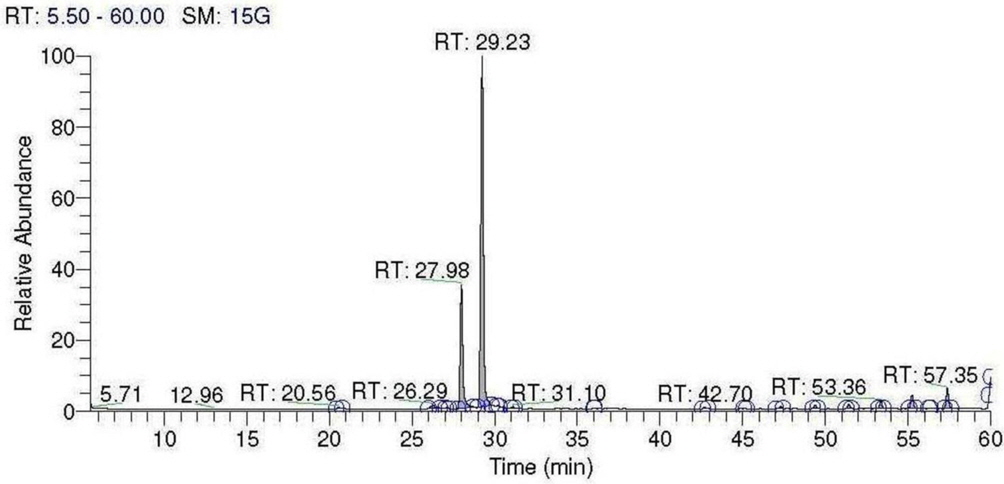
In GC-MS analysis, seven chemical compounds were identified in cinnamon oil which comprising of 97.92% of the total cinnamon oil. Cinnamaldehyde (66.74%) and eugenol (22.91%) were found main components of cinnamon oil, followed by Triacontane (3.30%), Heptacosane (2.10%), Penta tri acontane (1.12%), Camphene (1.02%) and Tetratetracontane (0.73%) were other minor constituents (Table 2, Fig. 5).
[Table 2.] GC-MS analysis of cinnamon oil
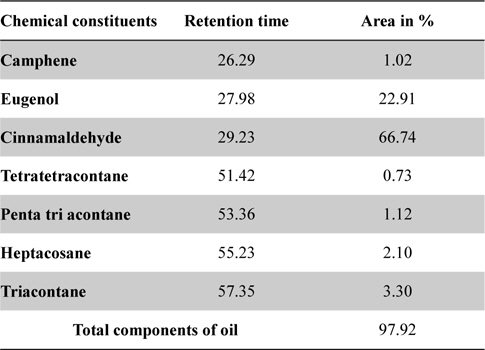
GC-MS analysis of cinnamon oil
The result of TLC bio-autographic/96-well microtiter plates confirmed the cinnamon leaves and oil has potential anticholinesterase activity. Cholinesterase inhibitors obtained from plant sources have been extensively studied for neurodegenerative disorder. These natural occurring compounds have provided convincing evidence of anticholinesterase activity in mild to moderate form of Alzheimer’s disease (Yang et al., 2003). It has been hypothesized that both AChE/BChE play critical role in maintaining chemical neurotransmitter (ACh) in the region of human brain. However, the ratio AChE/BChE label in certain region of brain is altered in due course of AD. These changes provide strong evidence of decline in label of acetylcholine in the region of dementia brain (Bhadra et al., 2012). This needs to design a suitable chemical substance which can optimize the therapeutic balance between AChE as well as BChE inhibition. The tested substances were shown more affinity towards AChE than BChE. This may be due to presence of chemical substance eugenol as constituent in methanol extract (Laekeman, 2011) and cinnamon oil. The incurred data clearly demonstrate the active principle contain tested substance have the potential cholinesterase activity and support the earlier evidence of claiming eugenol contain plant extract has possible anticholinesterase activity (Kumar et al., 2009).
The content of eugenol in cinnamon oil was standardized with RP-HPLC and identified through gas chromatography−mass spectrometry (GC−MS) technique. GC−MS technique is widely employed for identification and quantification of volatile compound present in essential oil (Bhadra et al., 2012). The variation of the different chemical compounds identified in cinnamon oil may be due to geographical location or soil condition (Rana et al., 2012). The identification of chemical compounds in cinnamon oil was corelated with data value of retention indices and mass spectra obtained from data library (Adams, 2001).
The present study highlights the eugenol as important constituent of