Andrographis paniculata (Burm. F.) Wall. Ex Nees is a traditionally known medicinal plant of Acanthaceae family, and andrographolide is quantitatively the major bioactive secondary metabolite of the plant identified to date. Amongst numerous plants of the Andrographis genus, Andrographis paniculata is the only one widely used for medicinal purposes, and it is also pre-clinically and clinically the most well studied one. Besides being well known as an Ayurvedic herb, Andrographis paniculata is also medicinally used in the traditionally known medical systems of China and Thailand. In Traditional Chinese Medicine, Andrographis paniculata is indicated for conditions of “heat,” particularly in the lungs, throat, and urinary tract, as well as for manifestations of “Fire Poison” on the skin, such as sores and carbuncles (Bensky and Gamble, 1986). Andrographis paniculata is also known as Cheonshimryeon in Korea and Chuan Xin Lian in China. Extracts of this plant parts and isolated andrographolides have been used to pharmacologically and experimentally verify its traditional usage for rheumatoid arthritis, inflammation, cold, fever, and diarrhea (Burgos et al., 2009; Chandrasekaran et al., 2010; Chandrasekaran et al., 2011; Shen et al., 2013). The WHO monograph on the plant published during 2003 mentions that its uses for prophylaxis and symptomatic treatments of upper respiratory tract infection, bronchitis, pharyngotonsillitis, lower urinary tract infections and acute diarrhea are supported by clinical data. Apart from these infectious diseases, diverse other traditionally known medicinal uses of the plant are also mentioned in pharmacopoeia of diverse traditional systems of medicine (WHO, 2003). Extensive efforts made during past few decades have identified not only broad spectrums of therapeutically interesting pharmacological properties of diverse types of Andrographis paniculata extracts, but also of andrographolide and other structurally unique bioactive constituents of such extracts (Chao and Lin, 2010; Jayakumar et al., 2013; Kumar et al., 2012; Mishra et al., 2007; Subramanian et al., 2012; Valdiani et al., 2012). Amongst them the ones dealing with anticancer and anti-inflammatory activities of extracts rich in andrographolide, or of pure andrographolide, have attracted the most attention of modern drug discoverers (Hidalgo et al., 2013; Lim et al., 2012). Although the numbers of clinical reports revealing, or reconfirming, therapeutic potentials of Andrographis paniculata extracts for treatment of inflammatory disorders have continued to increase during recent years, as yet no such report on pure andrographolide has appeared.
In the ayurvedic system of medicine currently widely practiced in Indian, Andrographis paniculata is often used in combination with other herbs and health care procedures for helping patients suffering from diverse spectrums of organ pathologies and mental health problems. It has been estimated that Andrographis paniculata is used in more than 50% of herbal compositions commercialized in India for hepatic disorders (Govindarajan et al., 2005). Modern Ayurvedic scholars often classify Andrographis paniculata as Rasayana herb useful for maintaining stomach integrity and regulating energy metabolism and immune functions (Govindarajan et al., 2005; Thakur et al., 2012a; Williamson, 2002). Although many Rasayana herbs are now pharmacologically classified as herbal adaptogens, so far only some scattered information on adaptogenic or anti-stress activity of Andrographis paniculata extracts and their bioactive constituents have appeared. Aim of this communication is to summarize available preclinical and clinical information suggesting their adaptogenic potentials, and to point out potential uses of andrographolide for identifying pharmacological targets and mechanisms involved in modes of actions of herbal adaptogens.
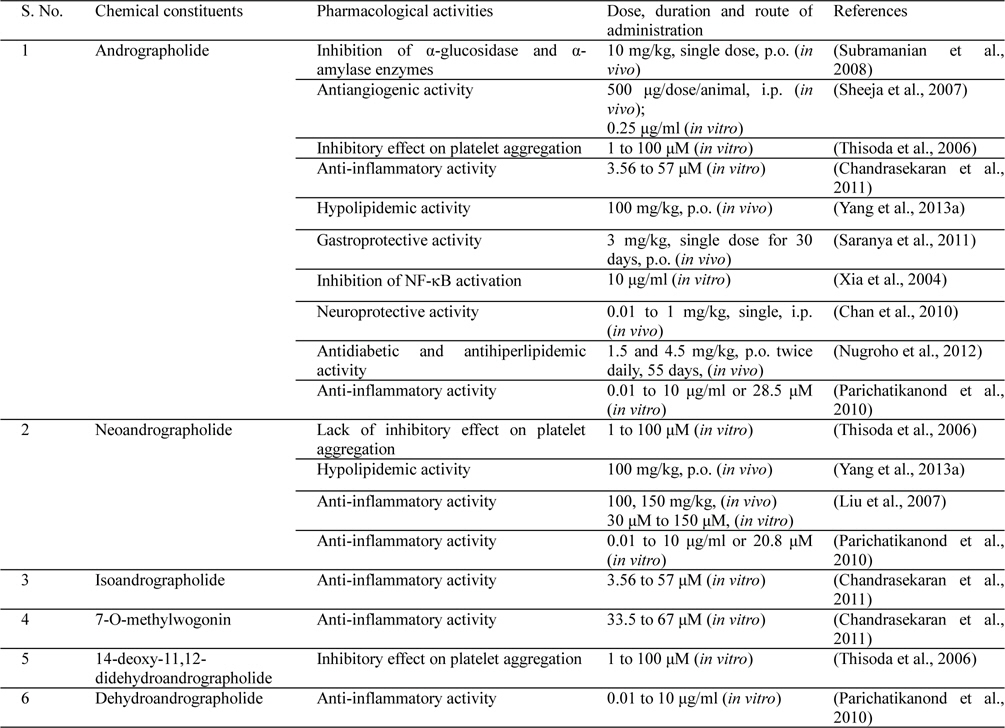
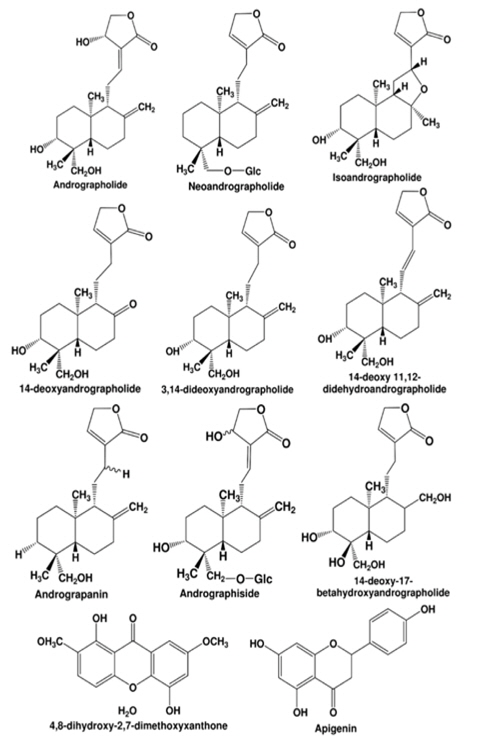
Andrographolide diterpinoids and 2'-oxygenated flavonoids are common chemotaxonomic markers of the Andrographis genus to which Andrographis paniculata belongs to (Koteswara Rao et al., 2004; Pramanick et al., 2007). Amongst more than 40 plants of this family, Andrographis paniculata is phytochemically as well as pharmacologically the most well studied one (Parixit et al., 2012). A number of diterpenoids and diterpenoid glycosides of similar carbon skeleton have been isolated from Andrographis paniculata. The most bitter compounds among them are andrographolide, neoandrographolide, isoandrographanolide, 14-deoxy 11, 12-didehydroandrographolide and andrograpanin. Other phytochemicals amassed by the plant are 14-deoxyandrographolide, andrographiside, deoxyandrographiside, homoandrographolide, andrographan, andrographon, andrographosterin, andrographidine G, stigmasterol, flavonoids, xanthones, phenol carboxylic acids and other antibacterial components (Hapuarachchi et al., 2013; Siripong et al., 1992; Subramanian et al., 2012). The leaves of Andrographis paniculata contain the highest amount of andrographolide (2.35%), while the roots (0.52%) and stem (0.35%) contain less amount of andrographolide in a 110 day old harvested Andrographis paniculata plant (Pandey and Mandal, 2010). andrographolide has highly bitter taste, is colorless crystalline in appearance, and possess a “lactone function” (Sharma et al., 1992).
Although flavonoids and other structurally diverse bioactive phytochemicals are encountered in medicinally used extracts of the plant, by far a vast majority of preclinical reports on such extracts concentrate mainly on their contents of andrographolide like labdane diterpinoids only. Structures of some the quantitatively major diterpinoids and their glycosides commonly encountered in such extracts are shown in Fig. 1. The relative contents of such bitter tasting molecules vary considerably in different parts of the plant, whereupon the content of andrographolide seems to be highest in its leaves (Jarukamjorn and Nemoto, 2008; Sharma et al., 1992).
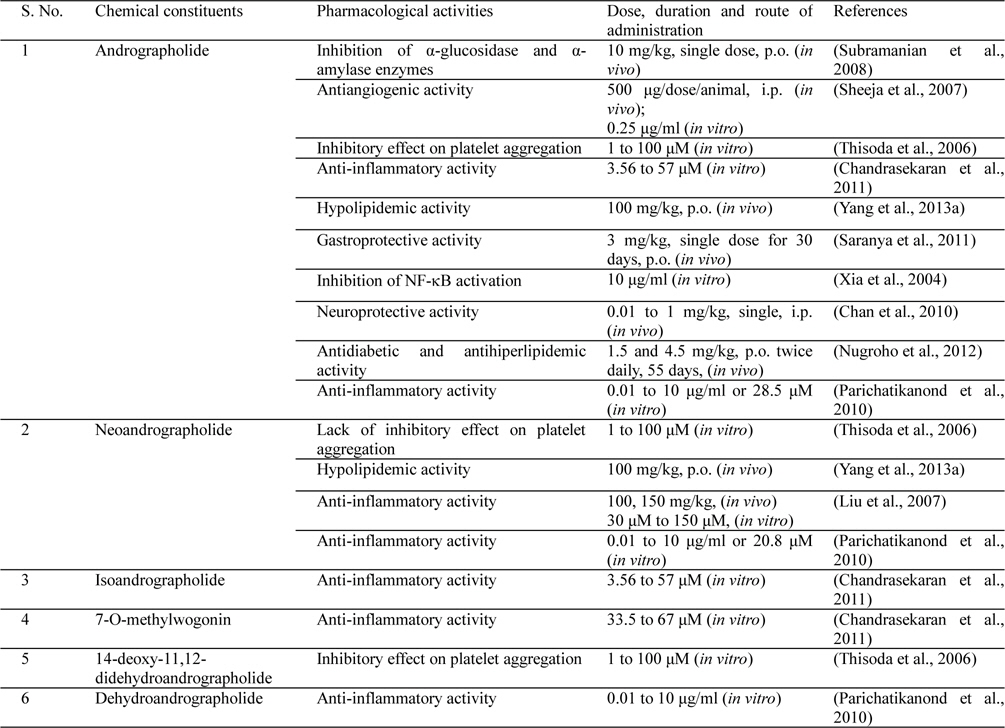
Andrographolide was first isolated in crystalline form during 1911 (Gorter, 1911), and the very first report suggesting that it is a bioactive constituent of Andrographis paniculata appeared during 1951 (Chakravarti and Chakravarti, 1951). Since then diverse of other structurally analogous diterpene lactones and their glycosides have been isolated from different parts of the plants, and several of them have also been reported to possess diverse therapeutically interesting bioactivities potentially useful for treatments of inflammatory disorders. Some such therapeutically interesting bioactivities of andrographolide and other diterpenes isolated from Andrographis paniculata are summarized in Table 1 and Fig. 2. It must be noted though, that apart from these diterpenes the medicinally used Andrographis paniculata extracts also contain diverse other constituents with antiviral, bactericidal, anti-oxidative and other therapeutically interesting bioactivities (Parixit et al., 2012), and reports on other secondary metabolites of the plant and their therapeutically interesting bioactivities still continue to appear (Hapuarachchi et al., 2013; Radhika et al., 2012b; Wu et al., 2008; Xu et al., 2010).
Available preclinical information on pure andrographolide and diverse types of Andrographis paniculata extracts (Parixit et al., 2012) strongly suggests that most probably andrographolide is the quantitatively major, but not the only, bioactive constituent of the plant. However, as yet little concentrated efforts have been made to define the roles of flavonoids and other structurally diverse bioactive constituents of the plant in the clinically observed efficacy of Andrographis paniculata extracts have yet been made, and therapeutic relevance of the experimentally observed brain function modulating effects of andrographolide, or of Andrographis paniculata extracts (Mandal et al., 2001; Radhika et al., 2012a), still remain at the best speculative only. Several recent observations made in cellular and other in vitro, or ex vivo, models strongly suggest that andrographolide is a neuro- or cerebro-protective agent, and that it could as well cross the blood brain barrier (Burgos et al., 2005; Carretta et al., 2009; Chan et al., 2010; Qin et al., 2006; Wang et al., 2004). It must be noted though, that oral bioavailability of andrographolide is poor (< 3%), and that after oral administration it is extensively bio-transformed to other molecules within the gastrointestinal tract itself (Guo et al., 2012; Panossian et al., 2000; Ye et al., 2011). Therefore, yet no very definitive statements on the role of andrographolide in the observed brain function modulating effects of the plant can yet be made.
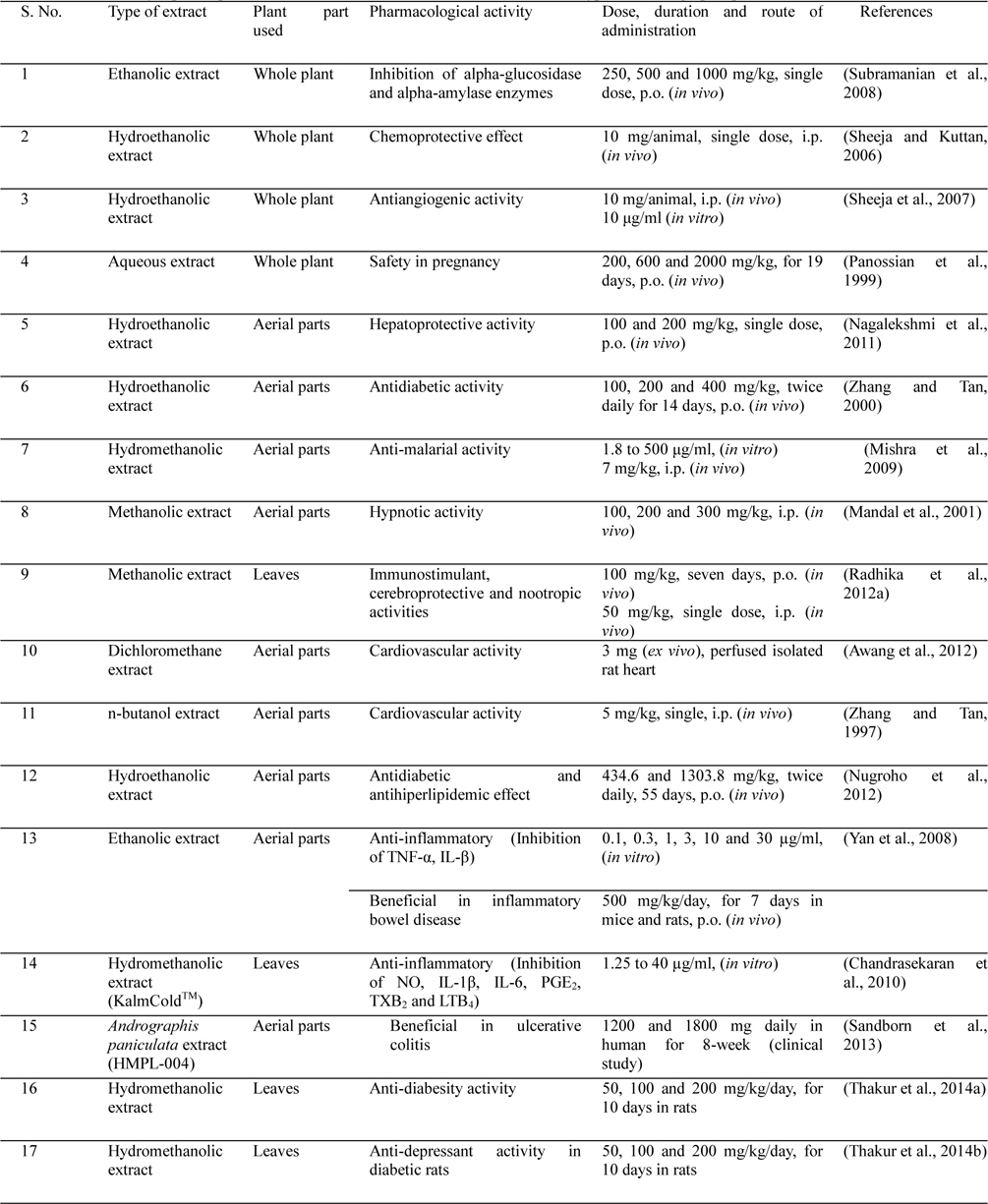
Similar, or analogous, is also the situation for many other therapeutically interesting pharmacological activities reported for diverse types of Andrographis paniculata extracts. Some such major ones reported during recent decades are summarized in Table 2 and Fig. 2. Although most such therapeutically interesting bioactivities have also been reported for pure andrographolide (Table 1), yet little concentrated effort gave been made to verify the possibility whether other bioactive constituents of Andrographis paniculata extracts modulates the efficacy and safety of andrographolide or not. Efforts to clarify the situation is not only necessary for appropriate analytical standardization of Andrographis paniculata extracts for therapeutic purposes, but also proper standardization of plant collection and processing procedures necessary for obtaining safe and more sustainable medicinal products from this wildly growing and well known medicinal plant.
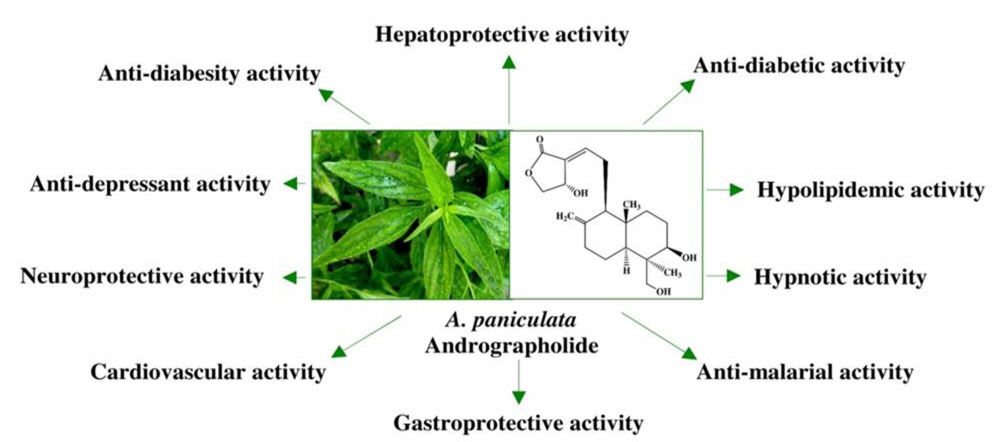
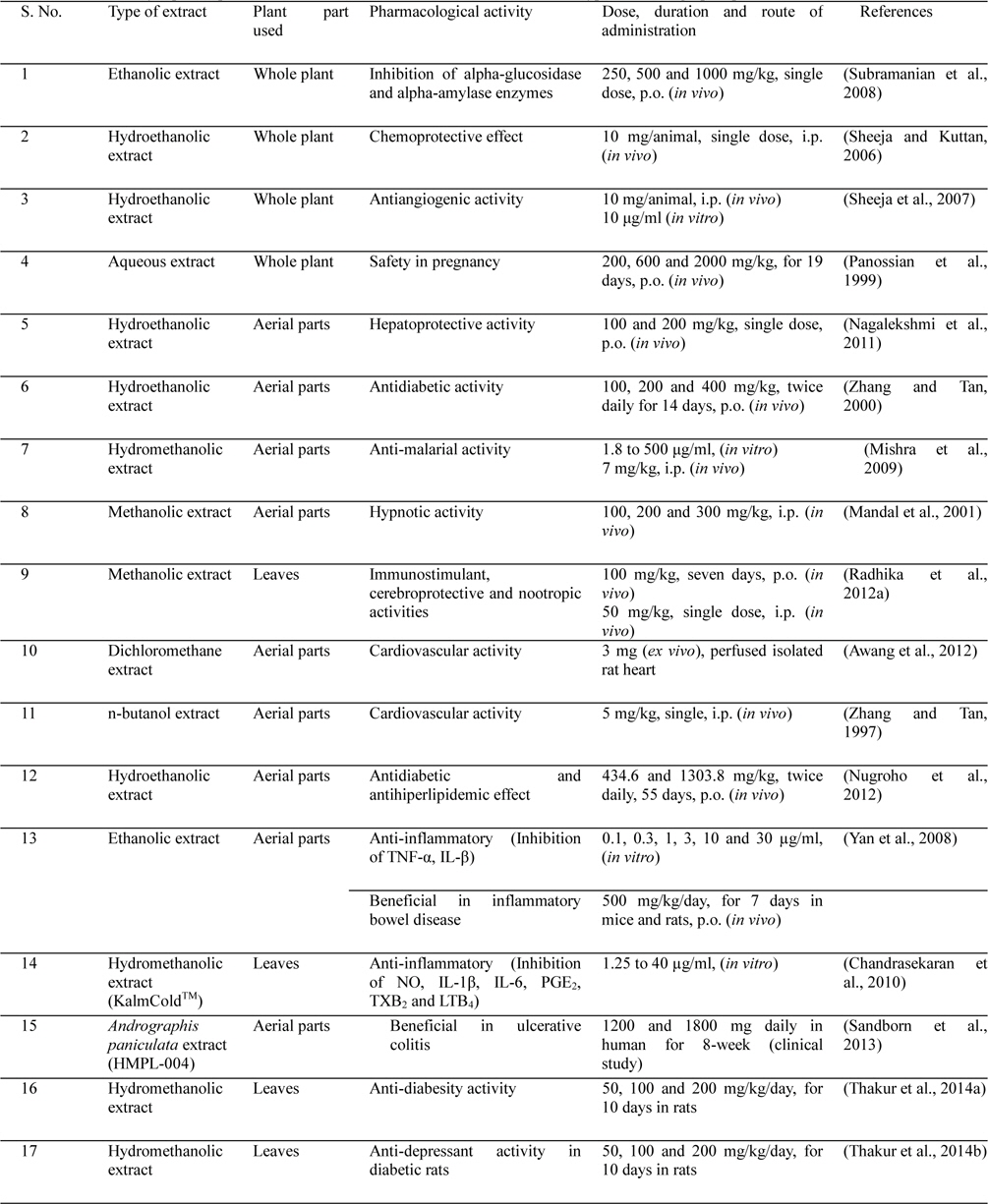
Andrographis paniculata has a broad range of pharmacological activities, such as antihyperglycaemic activity, hypolipidemic activity, antihyperlipidemic activity and antioxidant activity (Nugroho et al., 2012; Subramanian et al., 2008; Yang et al., 2013a; Zhang and Tan, 2000), cardiovascular activity (Awang et al., 2012; Thisoda et al., 2006; Zhang and Tan, 1997), hepatoprotective activity (Nagalekshmi et al., 2011; Chander et al., 1995), gastroprotective activity (Sandborn et al., 2013; Saranya et al., 2011), neuroprotective activity (Chan et al., 2010), antidiarrhoeal activity (Gupta, 1993), immunostimulatory activity (Calabrese et al., 2000; Iruretagoyena et al., 2005), antimalarial activity (Mishra et al., 2009; Najib Nik et al., 1999), antiviral activity (Wiart et al., 2005), anticancer activity (Li et al., 2007; Sheeja et al., 2007; Sheeja and Kuttan, 2006), anti-inflammatory activity (Chandrasekara et al., 2011; Liu et al., 2007; Parichatikanond et al., 2010; Pramanick et al., 2007; Sheeja et al., 2006; Xia et al., 2004; Yan et al., 2008) and protective effects in oxidative stress in brain associated with nicotine-induced toxicity (Das et al., 2009). It was demonstrated that the extract having some pharmacological activities related to central nervous system as indicated by its potentiating hypnotic and sedative activity (Mandal et al., 2001; Thakur et al., 2013), and immunostimulant, cerebroprotective and nootropic activities in normal and type 2 diabetic rats (Radhika et al., 2012a).
Literature survey also revealed that this medicinal plant was proven in preclinical and clinical studies for the prevention and treatment of common cold (Saxena et al., 2010), and upper respiratory tract infections (Coon and Ernst, 2004 and Ernst, 2004). Psychopharmacological studies were conducted with an extract of Andrographis paniculata. The extract produced a prolongation of the pentobarbiotone-induced sleeping time and lowered the body temperature in deferent experimental animal after single intraperitoneal injection. The extract also exhibited significant motor in-coordination and muscle relaxant activity. These finding reveal a potent brain function altering activities of Andrographis paniculata in rodent models and further detailed investigations are necessary (Mandal et al., 2001).
After oral administration of andrographolide, several metabolites were found in blood, urine, bile and the contents of the small intestine and stomach of rats which may be responsible for neuroprotective effect against cerebral ischaemia (Chan et al., 2010). The bioavailability of andrographolide and metabolites in brain may be due to crossing of brain-blood barrier (He et al., 2003). Neuroprotective effects against cerebral ischaemia with accompanying inhibition of microglia activation, possibly caused by the suppression of NF-ᴋB activation, leading to a reduction in the production of cytokines including TNF-α and IL-1β, and pro-inflammatory factors such as PGE2. andrographolide in permanent middle cerebral artery occlusion (pMCAO) induced ischemic rats demonstrated markedly reduction in IL-1β to a level of normal rats and markedly abolished the increase in TNF-α in ischemic rats. Andrographolide also abolished this increase in PGE2 levels. The NF-ᴋB is a transcription factor crucial for inflammatory gene expression. Activation of NF-ᴋB promotes nuclear translocation of p50 and p65 subunits. Marked inhibitory effect of andrographolide had shown in pMCAO induced nuclear translocation by inhibition of NF-ᴋB (Chan et al., 2010). Together with this report also several previous reported neuroprotective effects of andrographolide in a rat demonstrated reduced production of interferon (IFN)-γ and IL-2 in T lymphocytes induced by concanavalin- A (Burgos et al., 2005; Carretta et al., 2009). It also inhibited the production of TNF-α and IL-12 in lipopolysaccharide stimulated-macrophages (Qin et al., 2006). Previous reported study by Wang et al. (2004) demonstrated that andrographolide reduces the production of proinflammatory mediators including ROS, TNF-α, NO and PGE2 in microglial cultures. These findings argue against the notion that neuroprotective effects of andrographolide may result mainly from its metabolites (Wang et al., 2004).
In traditionally known medicinal systems of China, India, Thailand and many other Asiatic countries Andrographis paniculata has since long been known to be a safe and effective medicinal plant. Although a systematic review on safety and efficacy of diverse types of its extracts (Coon and Ernst, 2004) currently widely used for treatments of upper respiratory tract infections did identify a few mild and infrequent occurrence of adverse events, in general the extracts were evaluated as a safe and effective remedies. A recent review critically analyzing available information on safety and efficacy of the plant have pointed out some potential health hazards that might eventually arise from its uncontrolled widespread uses (Valdiani et al., 2012). Oral administration of Andrographis paniculata extract (up to 1000 mg/kg of body weight per day) for 65 days prior to mating and 21 days during mating, did not reveal any signs of its dose-dependent toxicity on reproduction and fertility (Allan et al., 2009), and it was found to be safe during pregnancy in doses up to 2000 mg/kg (Panossian et al., 1999). A series of in vitro toxicology studies conducted with a well standardized total extract of the plant did not reveal any genotoxicity potential of the extract (Chandrasekaran et al., 2009). A recent international pharmacovigilance report reveals though, that Andrographis paniculata derived drugs can sometimes cause hypersensitivity reaction in HIV positive patients (Farah et al.,2008). Since andrographolide and diverse other bioactive secondary metabolites of Andrographis paniculata possess diverse spectrums of immune function modulating activities, further more detailed studies will be necessary for proper assessment of the safety profile of its medicinally used extracts or of pure andrographolide in immune suppressed patients. It must be noted though, that even fairly high oral daily oral doses of pure andrographolide (up to 500 mg/kg/day for 3 weeks) is well tolerated by laboratory rodents (Bothiraja et al., 2012), and that its reported pharmacologically active oral doses administered with Andrographis paniculata extracts are several folds lower (Table 2). Taken together, these reports strongly suggest andrographolide is most probably one of the better tolerated secondary metabolite of the plant, and that it could be a promising starting point for identifying structurally and functionally novel immune function modulating drug potentially useful for treatments of diverse chronic diseases commonly caused, or associated with, persistent systemic inflammatory disorders.
The term adaptogen was first coined during 1940s by the Russian physician pharmacologist Lazarev to describe substances that increase body's nonspecific response to stress. This was based on observations made with bendazole i.e. a vasodilator developed in France and still commercialized in some other countries. Since then diverse modified or more precise definition of the terms have been proposed (Winston and Maimes, 2007), and herbalists trained in modern medical sciences now often use this term to pharmacologically characterize medicinal herbs with anti-stress activities. According to Brekhman (1980), the term adaptogen may be defined as follows: (a) an adaptogen must show a non-specific activity, i.e. increase in power of resistance against physical, chemical or biological noxious agents; (b) an adaptogen must have a normalizing influence independent of the nature of the pathological state; and (c) an adaptogen must be innocuous and must not influence normal body functions more than required. The very first more comprehensive pharmacological report pointing out that Ayurvedic Rasayana herbs have adaptogenic potentials appeared only during 1999 (Rege et al., 1999), and since then the list of Rasayana herbs with adaptogenic properties have increased consistently.
Since almost all better scrutinized Rasayana herbs possess also nootropics like cognitive function improving, anxiolytic and antidepressant activities in animal models, modern Ayurvedic scholars often classify them also as nootropic herbs (Kulkarni et al., 2012). A recent review on current status of herbal adaptogens lists more than 50 such plants, most of which are also well known to possess nootropic like activities (Pawar and Shivakumar, 2012). As a matter of fact, every pharmacologically better scrutinized Ayurvedic Rasayana herb have been found to possesses nootropic, anti-stress, immune function modulating as well as anti-oxidative properties. Although Andrographis paniculata is also a Rasayana herb, as yet little efforts have been made to verify the possibility that its clinically observed therapeutic benefits could as well be due to its anti-stress or nootropic activities. In other words, psychopharmacological activity profiles of Andrographis paniculata extracts and of pure andrographolide in animal models commonly used for identifying adaptogenic potentials of test agent (Mandal et al., 2001; Pawar and Shivakumar, 2011; Thakur et al., 2012b) still remains to be better defined.
A recent report have indicate though, that a methanol extract of Andrographis paniculata leaves possesses antioxidative, immunostimulating and nootropics like cognitive function improving activities in normal as well as diabetic rats (Radhika et al., 2012a). The observations reported in this communication add further experimental evidences in support of the conviction that Andrographis paniculata can also be pharmacologically a nootropic or adaptogenic herb. However, they do not allow any definitive statements on possible involvement of cognitive function improving effects of therapeutically used Andrographis paniculata extracts in their clinically observed efficacies, or on their active principles and modes of actions. Analogous are the cases for almost all Rasayana or other adaptogenic herbs. This is mainly because no very convenient and versatile animal model necessary for identification and pharmacological characterization of herbal adaptogens are yet available.
The fact that proper doses, formulations and combinations of Rasayana herbs has to be regularly administered for therapeutic purposes has been known to Ayurvedic practitioners since long. However, modern preclinical researchers while planning their experiments often neglect these facts. Actually, the situation has worsened since the evolution of postmodern concepts of molecular biology and pharmacology. Most preclinical researchers are now relying more on in vitro and cellular models, and often neglect the fact that repeated daily oral doses of any bioactive molecule must not necessarily have the same responses as those observed after their single oral, intravenous or intraperitoneally administered doses (Bond, 2002). The only major exceptions are toxicologists who consistently point out that safety of drugs can be better predicted only when the results of repeated dose toxicity studies are available.
Both experimental pharmacologists and toxicologist have since long been aware of the fact that proper dose finding experiments are necessary for more rationally predicting therapeutic potentials of bioactive molecules and their mixtures. However, it was only the observations of the toxicologists that eventually led to more widespread acceptance of the phenomenon of hormesis by almost all postmodern medical sciences (Calabrese, 2004; Zhang et al., 2008). Although the fact that hormetic dose response curves can be expected for numerous phytochemicals is now well established (Calabrese et al., 2010b), as yet the implications of hormesis in dictating the efficiencies of herbal remedies still continue to be largely neglected by experimental pharmacologists. This is mainly because the dose and time response of experiments necessary for defining their pre- and post-conditioning effects (Hausenloy and Yellon, 2009; Selzner et al., 2012) involved in their hormetic effects are often neglected or not conducted due to convenience, feasibility, and diverse other reasons. Resveratrol is just one example of such a well known and better scrutinized phytochemical with preconditioning and hormetic effects (Calabrese et al., 2010a; Das et al., 2005), and for which as yet no very definitive statements on its therapy relevant pharmacological dose range and treatment regimen has been assessed.
Numerous observations revealing that acute dose effects of drugs can be opposite to those identifiable after their repeated daily doses have led to suggestion of the term “paradoxical pharmacology” for describing this phenomenon (Bond, 2001; Page, 2011; Smith, 2012). Comparison of dose-response relationship observed after acute and repeated daily oral doses of an orally consumed bioactive agent, or drug, using the classically known animal models is a feasible means for avoiding misinterpretations of their therapeutic potentials. Unfortunately, reports on such comparative studies with herbal adaptogens in general, and Andrographis paniculata extracts and andrographolide in particular, are rare. Moreover, unlike for other known adaptogenic Rasayana herbs, yet only a few scattered reports on psychopharmacological activity profiles of Andrographis paniculata and its bioactive secondary metabolites have appeared. In view of the situation, a holistic psychopharmacological strategy based on available preclinical information on other adaptogenic herbs (Chatterjee and Kumar, 2012) is now being used in our laboratories for defining and comparing the therapy relevant pharmacological activity profiles of a well standardized Andrographis paniculata extract and of pure andrographolide. Observations made to date under this newly initiated project revealed that their anti-stress effects become detectable in rodent models only after their several daily oral doses (Thakur et al., 2013). Thus, it seems reasonable to assume that like many other Rasayana herbs, Andrographis paniculata is also an adaptogenic herb, and that andrographolide is one of its quantitatively major adaptogenic metabolite. Implications of such available preclinical and clinical information for more rationally discovering and developing drugs and phyto-pharmaceuticals are many folds. Some of them are pointed out in the following.
The WHO monograph and other recent reviews on medicinal phytochemistry and pharmacology of Andrographis paniculata and its bioactive secondary metabolites (Kumar et al., 2012; Mishra et al., 2007; Panossian and Wikman, 2013; Subramanian et al., 2012; Valdiani et al., 2012; WHO, 2003) have summarized their diverse therapy relevant bioactivities and point out that andrographolide must not necessarily be the only therapeutically interesting secondary metabolite of the plant. A recent draft of the assessment report of European Medicine Agency (published on 15th January 2013) on currently available preclinical and clinical information on Andrographis paniculata concludes (EMEA/HMPC, 2008):
“Due to the lack of appropriate studies, the requirements for well-established use are not fulfilled. The evidence of traditional use (long-standing use of preparation(s) with specific posology) is considered insufficient.”
It must be noted though, that the use of the term “appropriate studies” in this, and numerous other earlier analogous reports is mainly due to critical analysis of available information evolving from preclinical and clinical studies planned and conducted according to the reductionist and already known pharmacological target directed reductionist principles of modern medicine. Hereupon little attention were paid to the fact that many known bioactive secondary metabolites of Andrographis paniculata are pharmacologically pleiotropic, and that by virtue of their chemical reactivity and redox potentials they can modify the biological functions of numerous of endogenous macro- and macro-molecules of critical importance for health maintenance.
It cannot be overemphasized that therapeutic potentials of Andrographis paniculata extracts, or for that matter of any drug or phyto-pharmaceutical, can be properly judged only when due attention is paid to their pharmacokinetic characteristics, metabolism, and distribution pattern. Available information on such characteristics of andrographolide and a few other known constituents of Andrographis paniculata strongly suggest that after their oral doses their observed blood levels are much below to those necessary for observing their bioactivities in cellular and other in vitro models commonly used for understanding their modes of actions or therapeutic potentials (Panossian et al., 2000; Yang et al., 2013b). Therefore, it seems reasonable to assume that either the biological effects of the extracts and their constituent observed in such models are not good predictors of their therapeutic potentials, or that appropriate bioavailability data on therapy relevant bioactive constituents of therapeutically used Andrographis paniculata extracts have not yet been generated. However, since andrographolide and constituents of Andrographis paniculata extracts are extensively metabolized after its oral intake, it is possible that circulating metabolites of andrographolide and other constituents of such extracts are involved in their observed therapeutic efficacies in controlled clinical trials, or that therapeutically interesting bioactivities of such extracts and andrographolide observed after their oral doses in animal models are due their actions inside the gastrointestinal tract only.
Importance of gastrointestinal functions in regulation of energy metabolism and other bodily and mental functions have been known since long, and it is now becoming increasingly apparent that hereupon the gut microbiota ecology plays a crucial role (Rook et al., 2013; Thakur et al., 2014). Since antimicrobial, antiparasitic, cytoprotective, and spasmolytic activities of Andrographis paniculata extracts are known, and maximum possible gastric ulcer preventive effects of andrographolide has been detected after daily treatments with its oral doses as low as 3 mg/kg/day (Saranya et al., 2011), it seems reasonable to assume that its oral intakes must alter not only gut microbiota ecology, but also the functions of the gastrointestinal tract. Therefore, it can be expected that such effects of Andrographis paniculata extracts are also involved in their clinically observed symptomatic relief in patients suffering from diverse pathologies diagnosed according to the criterion commonly used in controlled clinical trials.
Almost all medical conditions against which therapeutic efficacies of Andrographis paniculata extracts have been demonstrate in properly controlled clinical trials are triggered by, or are associated with, systemic inflammation. Since central sensitivities to external triggers or internal bodily signals are altered in all such conditions, many such conditions are now grouped under the common heading “central sensitive syndrome” (Yunus, 2007, 2009). Such is the case for common cold or upper respiratory tract infections also (Smith, 2012, 2013), against which most properly controlled clinical trials with Andrographis paniculata extracts have been conducted to date (Panossian and Wikman, 2013; Subramanian et al., 2012). Since animal behavioural models are reliable indicators of cognitive functions and central sensitivity, and oral efficacy Andrographis paniculata extracts in some such models have been observed, it can be expected that at least some traditionally known medicinal uses of the plant as an adjuvant or bitter tonics could as well be due their adaptogenics effects on brain functions. However, more detailed studies with purified andrographolide and other extractable bioactivity of metabolites of Andrographis paniculata are still needed for experimentally verifying this possibility or to make a more definitive statement on such possibilities.
Although like almost all herbal adaptogens and Ayurvedic Rasayana herbs, Andrographis paniculata extracts also possess antioxidative and immunomodulatory activities (Govindarajan et al., 2005; Thakur et al., 2012a; Williamson, 2002), our current knowledge on their bioactive constituents and biological interactions between them still remain to be limited, or at the best speculative only. Amongst structurally diverse phytochemicals identified to date from the plant, andrographolide has attracted the most attention of modern drug discoverers for obtaining structurally novel therapeutic leads against cancer, diabetes and diverse other diseases involving systemic inflammation, Consequently numerous derivatives and analogues of andrographolide are now being synthesized and subjected to diverse bioassays (Zhou et al., 2013). However, several other structurally analogous diterpene lactones of potential therapeutic interest are also known (Table 1), and the presence of anti-inflammatory flavonoids in therapeutically used Andrographis paniculata extracts has also been reported (Chandrasekaran et al., 2010; 2011). Thus, it is apparent that theoretically diverse therapeutic benefits of the plant can be obtained by uses of diverse processing and standardization procedures. However, yet no very systematic efforts have been made to more rationally exploit such possibilities.
Although more detailed analytical data on many clinically or pre-clinically tested Andrographis paniculata extracts are seldom available, most of them have been reported to contain varying concentrations of andrographolide. Amongst diverse therapeutically interesting pharmacological activities of purified andrographolide reported to date, the ones dealing with its gastro protective and antidiabetic potentials were observed after its oral doses (Table 1), and analogous, or similar, effects of Andrographis paniculata extracts have also been reported in animal models (Table 2). However, daily 1.5 to 4.5 mg/kg oral doses of pure andrographolide were sufficient for observing its efficacies, whereas when administered with Andrographis paniculata extracts its much higher doses were necessary for obtaining similar or analogous effects. Such analysis of available information on preclinical data on andrographolide and Andrographis paniculata extracts strongly suggest that some other bioactive constituents of the tested extracts actually reduces, or antagonizes, the efficacy of Andrographolide. Clarification of such discrepancies is not only necessary for judging the therapeutic possibilities offered by the plant, but also for appropriate analytical standardization of Andrographis paniculata extracts commercialized by herbal industries and often used in numerous herbal formulations.
It has been reported indeed that even the dried powder of the aerial part of Andrographis paniculata contains 5.45% andrographolide, and that administration of 600 to 1800 mg/day of such powder to type-2 diabetic patients significantly reduces their blood HbA1c contents by 5.46% and fasting serum insulin contents by 20.93% (Agarwal et al., 2005; Michelsen et al., 2013). Therefore, it seems reasonable to assume that analytically and pharmacologically well standardized Andrographis paniculata extracts could be a more holistic and realistic approach for combating diabesity, than reductionist and target oriented approaches commonly used for such purposes by most modern drug discoverers. Diabesity is now well recognized by WHO as the epidemic of the 21st century which is spreading more rapidly in developing Asiatic countries like India and China where Andrographis paniculata can be easily and more economically cultivated. Since diabesity is the major risk factor for almost all major non communicable diseases affecting all bodily and mental function regulated by endogenous immune and nervous systems (Colagiuri, 2010; Schmidt and Duncan, 2003; Wolowczuk et al., 2008), appropriate uses of adaptogenic potentials of Andrographis paniculata and its metabolites could as well be a feasible means for prevention of many health problems caused by, or associated with, this epidemic.
It cannot be overemphasized though; achieving such goals is possible only when a convenient and therapy relevant bioassay models suitable for regular quality control of Andrographis paniculata extracts is available. At present, no such model is available for Andrographis paniculata extracts, or for any other known popular and medicinally widely used adaptogens. Since andrographolide seems to be the major bioactive secondary metabolite of the plant, appropriate knowledge on its adaptogenic potentials could as well lead to some such bioassays. Unfortunately, modern herbal researchers have yet paid little attention to such possibilities.
It is apparent from (Table 2) that diverse types of Andrographis paniculata extracts were used in different studies and that orally administered doses tested in animal models also varied enormously. Some such doses used were even much higher than the highest one used in earlier published toxicological studies (Allan et al., 2009; Burgos et al., 1997). A recent report on toxicity of pure andrographolide reveal that its 500 mg/kg/day daily doses administered for 21 days are well tolerated by laboratory rats and that 250 mg/kg/day is high enough to observe its effects on white blood cell and lymphocyte counts (Bothiraja et al., 2012). Authors of this report interpret these findings as an indicator of its immunostimulant activity reported earlier using in vitro models (Basak et al., 1999). However, even none of many such toxicological studies have paid any attention to the pharmacologically effective doses of the extracts or of andrographolide necessary for proper assessment of their safety margins, and demonstrate only that they are toxicologically safe even after their very high repeated daily oral doses.
Apart from such negligence, most herbal researchers and modern drug discoverers consistently neglect the fact that gut microbiota and the gut-brain axis plays an important role in regulating not only metabolism and immune functions, but also cognitive and other mental functions (Rook et al., 2013). Since all orally consumed phytochemicals are metabolized inside the gut by the microorganisms present there, their blood levels are not always very reliable indicators of their potential actions on brain functions (Zhang et al., 2012). Flavonoids are some such well known examples of brain function modulators for which no correlation between their blood levels and bioactivities are found (Vissiennon et al., 2012). Since numerous flavonoids are also encountered in Andrographis paniculata, or for that matter in almost all adaptogenic and other plants, the possibility that their efficacies observed after oral administrations is due to their actions inside the gastrointestinal must be given due considerations. Moreover, it cannot be overemphasized that andrographolide and numerous other extractable secondary metabolites of Andrographis paniculata possess antimicrobial and antiparasitic effects. Consequently, it can be expected that they alter the gut microbial ecology regulating metabolic, immunological as well as brain functions (Cani and Delzenne, 2009; Dinan and Cryan, 2012).
These and numerous other well were known biological and pharmacological facts on medicinal herbs and their secondary metabolites (Bone and Mills, 2012; Brencic and Winans, 2005) have often been neglected by modern herbal researchers interested in properly understanding of therapeutic potentials of Andrographis paniculata, or for obtaining therapeutic leads from it. Moreover, almost as a rule the pharmacological strategies and models evolving during the second half of the 20th century from pharmaco-centric and reductionist drug discovery principles still continue to be broadly used for such purposes. Detailed discussion on the consequences of such practices has been nicely pointed out in several recent comprehensive reviews on therapeutic potentials Andrographis paniculata and other adaptogens (EMEA/HMPC, 2008; Samuelsson and Bohlin, 2009; Subramanian et al., 2012; Valdiani et al., 2012). In view of the fact, that Andrographis paniculata extracts and their bioactive constituents are pharmacologically pleiotropic and polyvalent, at present more holistic approaches and widespread uses of conventionally known animal models (Chatterjee and Kumar, 2012) could be a more rational approach for discovering drug leads from this easily cultivable plant, or for properly understanding the therapeutic possibilities offered by it.
As reflected by the concluding remarks of the expert committee of European Medicine Agency cited before, most expert opinions on therapeutic potentials of Andrographis paniculata and their bioactive constituents are ultimately judged by the regulatory authorities only by the results and reproducibility of the observations made in appropriately controlled clinical trials according to the postmodern concepts of modern medicine. Despite such reports and evaluations, numerous holistic medical practitioners, trained or not in herbal medical sciences, are still very convinced that appropriate uses of Andrographis paniculata extracts together with other health care practices is an useful means for treatments or prevention of several medical conditions not preventable and treatable by affordable therapeutic measures offered by modern drugs and other therapeutic possibilities. To our judgments, this difference in opinion and practices is mainly due to the difference in doses and treatment regimen of diverse types of formulations of the plant used in controlled clinical trials and by holistic medical practitioners.
In general, the holistic medical practitioners recommend low doses and prolonged regular uses of herbal preparations containing Andrographis paniculata extract as one of the active ingredient, whereas almost as a rule the clinical trials are conducted with much higher doses of extracts alone and that too during shorter observation periods. All known adaptogenic substances are modulators of endogenous hormones and biological mechanisms involved in homeostasis (Pawar and Shivakumar, 2012), and their dose response curves in bioassays are inverted U- or J-shaped. Therefore, appropriate dose response studies with Andrographis paniculata extracts and andrographolide could not only help resolve the still ongoing controversial discussions on their therapeutic potentials, but also will be useful for designing of clinical trials necessary for taking decisions on their appropriate dose ranges and treatment regimens necessary for obtaining therapeutic benefits from their adaptogenic properties.
Since andrographolide is quantitatively major bioactive constituent of Andrographis paniculata extracts and its pharmacological activity profile is qualitatively similar to those of medicinally used extract, detailed dose response studies in appropriate cellular or animal models could be an initial first step for achieving such goals. Such appropriate models can be identified by proper use of several already known therapies relevant information on andrographolide. One such information relevant for its unspecific adaptogenic activities is that it is a chemically reactive molecule and can covalently react with diverse endogenous micro- and macro-molecules involved in energy metabolism and other regulatory functions (Woo et al., 2008; Xia et al., 2004). Another such information available for andrographolide is it high affinity to bitter taste receptors (Behrens et al., 2009). Although such receptors are known to exist throughout the gastrointestinal tracts and other bodily organs, their biological functions remain speculative only (Clark et al., 2012). Therefore, uses of andrographolide as a covalently binding pharmacological tool and appropriate animal models could a reasonable approach for clarifying potential involvement of such and other receptors or targets in the observed therapeutic efficacies of Andrographis paniculata extracts.
Availability of an animal model for estimating pharmacologically interesting dose range duration of action of andrographolide will be useful not only for obtaining better estimates of its therapeutic potentials, but also could lead to the identification of novel, or as yet neglected, pharmacological targets and mechanisms potentially useful for obtaining structurally and functionally novel drug leads. Moreover, since numerous ayurvedic Rasayana herbs and other adaptogens possess overlapping, or analogous, pharmacodynamic and pharmacokinetic profiles known for Andrographis paniculata, such models could also be useful for better understanding of their sites and modes of actions, or for identifying their therapy relevant pharmacological activity profiles. In any case, availability of such a model could be instrumental for identifying other bioactive constituents of medicinally used Andrographis paniculata extracts modulating the actions of andrographolide. Such knowledge is urgently needed of more rational analytical as well as pharmacological standardization of Andrographis paniculata extracts for medicinal or health care purposes.
Available information on pharmacological activity profiles of numerous herbal adaptogens and some of their bioactive constituents clearly reveal that all of them alter behavioral responses of laboratory rodents to diverse external stimuli, and that their effects are mediated by their actions on the hypothalamic pituitary adrenocortical system regulating homeostasis. This system is also involved in thermoregulation and body temperatures changes occurring during numerous external stimuli and such changes can be conveniently quantified by body temperature measurements. Attempts are now being made in our laboratories to identify rodent models suitable for quantifying the effects adaptogens on thermoregulatory process. Preliminary observations made in our laboratories indicate that like many other adaptogens, daily oral treatments of rodents with andrographolide or a medicinally used Andrographis paniculata alters hyperthermia induced by daily handling, or by subjecting them to transient mild stressful situations. However, neither qualitatively, nor quantitatively, the observed effects of the extract were identical to that of pure andrographolide. These observations encourage us suggest that rodent models commonly used for gaining more precise information on thermoregulatory processes could as well be useful ones for getting therapy relevant pharmacological information not only for Andrographis paniculata extracts, but also for other Ayurvedic Rasayana herbs and adaptogens.
Urgent therapeutic necessity of such efforts cannot be overemphasized. Despite extensive efforts and considerable progress, the mental health problems still continue to be major challenges for modern as well as traditionally known medical sciences. Since all such problems accompany, or aggravate the progression of, almost all major medical conditions (including diabesity), and several controlled clinical trials have repeatedly demonstrated clinical efficacies of Andrographis paniculata extracts against diverse central sensitivity syndromes, it seems reasonable to suggest that proper exploitation of this traditionally known medicinal plant is a promising starting point for obtaining novel therapeutic leads against diverse spectrums of psychopathologies encountered in almost all societies around the globe. To our judgments, the old fashioned and more holistic pharmacological strategies could be the more realistic ones for such ventures.