New precursors, poly(acrylonitrile-co-crotonic acid) (poly(AN-CA)) and poly(acrylonitrileco- itaconic acid-co-crotonic acid) (poly(AN-IA-CA)) copolymers, for the preparation of carbon fibers, were explored in this study. The effects of comonomers with acidic groups, such as crotonic acid (CA) and/or itaconic acid (IA), on the stabilization of nanofibrous polyacrylonitrile (PAN) copolymers were studied. The extent of stabilization, evaluated by Fourier transform infrared spectroscopy, revealed that the CA comonomer could retard/control the stabilization rate of PAN, in contrast to the IA comonomer, which accelerated the stabilization process. Moreover, the synthesized PAN copolymers containing CA possessed higher Mv than those of the IA copolymers and also showed outstanding dimension stability of nanofibers during the stabilization, which may be a useful property for improving the dimensional stability of polymer composites during manufacturing.
Carbon fibers are finding increased uses in a variety of applications because of their high tensile strength and modulus combined with their light weight, which make these fibers attractive for high volume applications ranging from sporting goods to aircraft structures [1]. It is well known that carbon fiber can be made from various precursors, such as polyacrylonitrile (PAN), rayon, pitch, etc., for industrial production at present [2]. Among these materials, PAN-based precursors are preferable since the resulting carbon fibers have relatively high strength and elongation, and have very low volume fraction of voids [3-5]. However, the use of pristine PAN homopolymer is restricted due to its low spinnability and stabilization [6,7]. An important strategy to improve the processability of pristine PAN is to incorporate small amounts of comonomers containing a carboxyl group, such as acrylic acid (AA), itaconic acid (IA), or methacrylic acid (MAA) into PAN during polymerization. The use of acidic comonomers is indeed preferable for significant reduction of the initiation temperature of cyclization; such addition also promotes the stabilization of PAN. In this study, we have evaluated PAN copolymers containing crotonic acid (CA), a structural analog of MAA, as new precursors for carbon fiber; these new materials are compared with PAN homopolymer and poly(AN-IA) copolymer as controls. The effects of carboxylic acid on the stabilization of PAN copolymers were examined. The synthesized PAN homopolymer, poly(AN-IA), poly(AN-CA), and poly(AN-IA-CA) copolymers were electrospun into fine fibers; this process was followed by stabilization. Structural evaluation was performed by Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), and X-ray diffraction (XRD) analysis.
2.1. Synthesis of PAN homopolymer and poly (AN-CA), poly(AN-IA), poly(AN-IA-CA) copolymers
PAN homopolymer and poly(AN-IA), poly(AN-CA), poly(AN-IA-CA) copolymers were prepared using a conventional free-radical polymerization technique. Briefly, 3.92 g of acrylonitrile (AN) (98.0 wt%), 0.08 g of CA (2.0 wt%), and 12.4 mg of azobisisobutyronitrile (AIBN, 0.2 mol% of monomers), were stirred in 16 g of dimethyl sulfoxide (DMSO) under nitrogen atmosphere at room temperature for 30 min followed by reflux at 60℃ for 24 h. The synthesized poly(AN-CA) copolymer was precipitated in deionized water, and then washed with deionized water and methanol in sequence. The solid product was recovered by filtration and dried under vacuum at 40℃ for 24 h. Similarly, poly(AN-IA) copolymer was also synthesized. In the case of poly(AN-IA-CA), the total comonomer concentrations of IA (fIA) and CA (fCA) were controlled to be ca. 2.0 wt%. Each comonomer was kept at a concentration of 1.0 wt%. PAN homopolymer was synthesized using a similar procedure in the absence of comonomers.
2.2. Stabilization of electrospun PAN homopolymer and its copolymer nanofibers
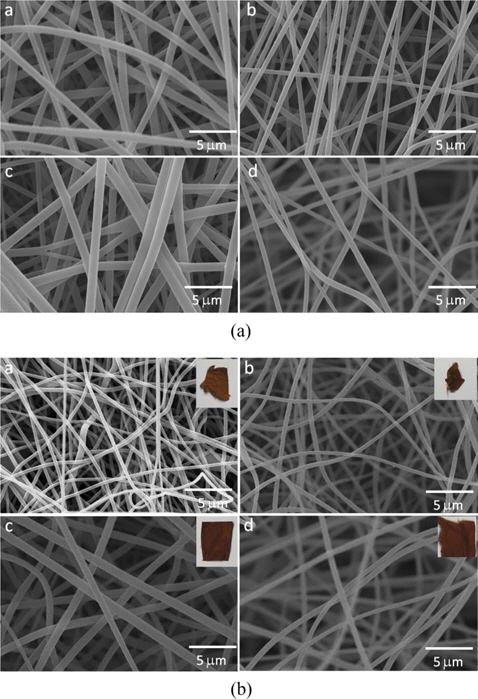
All the synthesized polymers were dissolved in DMF under magnetic stirring with a polymer content of 9.0 wt%. The solution was supplied through a 10-mL syringe having a plastic tip with an inner diameter of 0.6 mm. Electrospinning [8] was carried out at 8 kV using a NanoNC power supply with a tip-to-collector distance of 15 cm at about 23℃-27℃ and relative humidity of 45%-55%. The electrospun fibers were collected on an aluminum foil and dried under vacuum at room temperature. The stabilizations of the electrospun PAN homo- and copolymer nanofibers were performed by increasing from room temperature to 280℃ at a heating rate of 1.0℃/min; samples were kept at 280℃ for 1 h under aerobic condition [9,13].
The Ubbelohde viscometry method was used to measure the viscosity average molecular weight (
where [η] is the intrinsic viscosity obtained by extrapolating the concentration-reduced viscosity plot to zero concentration.
The synthesized polymers were characterized by 1H NMR spectroscopy (FT-NMR, JNM-EX400, 400 MHz) in DMSO-d6 solvent. The morphologies of the as-spun and stabilized nanofibers were observed under a JEOL JSM-5900 scanning electron microscope (SEM) after sputtering the samples with platinum for 120 s. Thermogravimetric analyses (TGA) were conducted from room temperature to 800℃ using a TA Q600 at a heating rate of 10℃/min. XRD patterns were recorded on a Rikaku X-ray diffractometer (Cu Kα radiation) with a scanning rate of 4°/min.
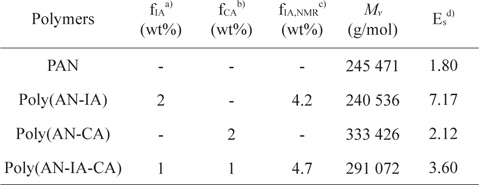
Poly(AN-CA) and poly(AN-IA-CA) copolymers containing CA as a new precursor for carbon fiber were synthesized by solution polymerization technique. The experimental conditions and characteristics of the synthesized polymers are summarized in Table 1. It was noteworthy that the PAN copolymers containing CA synthesized in this study showed higher
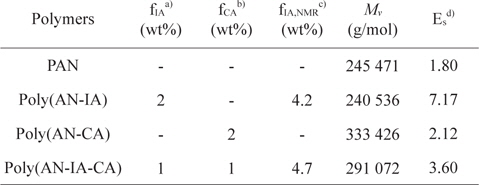
Synthesis and characteristics of PAN homopolymer, and poly(AN-IA), poly(AN-CA), poly(AN-IA-CA) copolymers
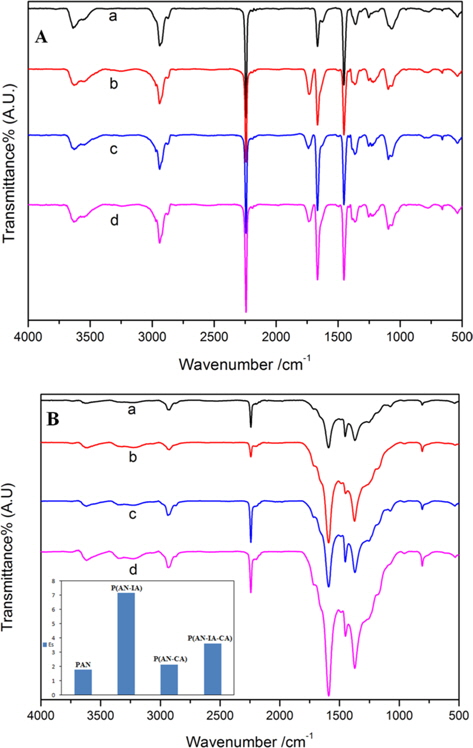
Fig. 2 shows the FT-IR spectra of the PAN homopolymer and its copolymers before Fig. 2(A) and after Fig. 2(B) heating at 280℃ for 1 h. As can be seen in Fig. 2a, the band at 2245 cm-1 was due to a characteristic stretching vibration of C≡N in the synthesized PAN homopolymer. For the case of the PAN copolymers (b, c, d in Fig. 2(B)), the C=O group was observed at 1738 cm-1, due to an incorporated comonomer containing carboxylic acid groups. Importantly, after heat treatment, the 1738 cm-1 band of the C=O group disappeared, and the C≡N bond at 2245 cm-1 decreased significantly, indicating that a cyclization reaction had occurred [13]. Moreover, a new peak at 1589 cm-1 appeared due to the C=C, C=N bond, which suggests the formation of a ladder structure [14]. As the stabilization proceeded, the 2245 cm-1 band of the nitrile groups decreased in intensity. The 1595 cm-1 band continued to increase, showing an enhanced stabilization reaction [15]. Here, the Es parameter was defined to evaluate the extent of stabilization:
where A is the absorbance, defined as A = log(To/T); To and T are the transmittances at baseline and maximum, respectively. In accordance with Lambert-Beer’s law: A = abc, where a is the molar absorption coefficient, b is the thickness of the film (cm), and c is the mole concentration (mol/cm). Since the absorbances (A1595 cm-1, A2245 cm-1) of the two peaks at the 2245 and 1595 cm-1 bands were determined using the same fiber, namely, a fiber with the same value of b, the ratio of the absorbance of these two bands is approximately equal to the ratio of the content of the generated C=N groups to the residual C≡N groups. The inset in Fig. 2b shows the Es values of the PAN homopolymer and of its copolymers with different weight ratios of CA and IA comonomer contents. The Es value of the PAN copolymers was higher than that of the PAN homopolymer. That is, the poly(ANIA) copolymer had an Es value higher than that of the other copolymers. Moreover, the Es value of the poly(AN-IA-CA) copolymer was somewhat larger than that of the poly(AN-CA) copolymer. This indicates that the CA comonomer can retard/control the extent of stabilization of PAN; this is in contrast to the case of the IA comonomer, which accelerates the stabilization. It is most important to control the rates of physical and chemical changes due to the presence during the stabilization of a variety of exothermic chemical reactions, including cyclization, dehydrogenation, oxidation, crosslinking, and fragmentation. Among these processes, the cyclization reactions, which convert the polymer chains into an infusible stable ladder structure, were of highest importance, playing a key role in the preparation of the carbon fibers [16].
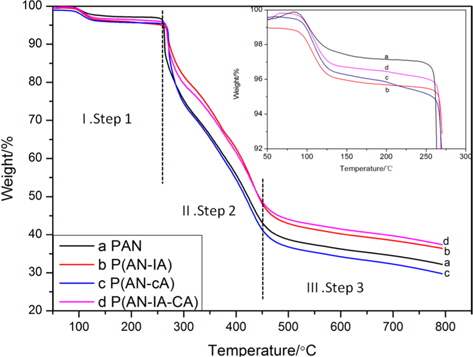
In order to investigate the thermal degradation behaviors of the synthesized PAN homopolymer and its copolymers, TGA analysis was carried out. Fig. 3 shows the TGA results for the synthesized PAN homopolymer, and for the poly(ANIA), poly(AN-CA), poly(AN-IA-CA) copolymers. TGA data can be separated into three main steps based on the degree of weight loss. The first step was prior to 260℃, during which the weight loss was tiny and cyclization occurred. Clearly, the PAN copolymers had higher levels of weight loss than did PAN homopolymer. It was apparent that IA and CA as acidic comonomers accelerated the extent of cyclization during the stabilization, which also led to the lower Es value (inset of Fig. 2b). The second step was around 260℃-450℃, during which the curves exhibited a sharp decline, mostly due to dehydrogenation [16,17]. After the formation of a stable ladder structure via cyclization, the PAN homopolymer and poly(AN-CA) copolymer showed higher levels of decomposition. This suggests that the greater cyclization for the case of the poly(AN-IA) copolymer resulted in greater thermal stability. However, the poly(AN-IACA) copolymer with the CA of 1.0 wt% showed a thermal stability similar to that of the poly(AN-IA) copolymer, indicating that the CA comonomer improved the thermal stability during the second step (Fig. 2). During the third step, above 450℃, the weight loss tended to be stable. Furthermore, the poly(ANIA) and poly(AN-IA-CA) copolymers exhibited higher residues at 800℃ than did the PAN homopolymer or the poly(AN-CA) copolymer.
The typical XRD pattern of the as-spun PAN homopolymer showed a strong peak at 2θ = 17°; its copolymer nanofibers showed relatively weak peaks around 2θ = 24° (for the PAN homopolymer) and 29° (for the PAN copolymers); these last two peaks were assigned to the (100) and (010) crystalline planes of the pseudohexagonal lattice. Compared to the PAN homopolymer, the peak at 2θ = 17° in the case of the PAN copolymers was relatively weak. This indicates that there were fewer intermolecular nitrile group pairings in the PAN copolymers because of the random/irregular arrangement of the molecular chains [14]. After stabilization of the poly(AN-IA) copolymer fibers, the intensity of the main peak at 2θ = 17° nearly disappeared (data not shown). Similar observations have been reported in other studies [14]. On the other hand, poly(AN-CA) and poly(AN-IACA) copolymer fibers still showed weak diffraction, suggesting that the incorporated CA comonomers were able to sustain the crystal structure of the polymers.
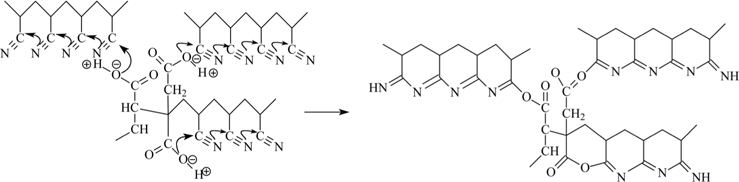
Scheme 1 shows the cyclization in the poly(AN-IA-CA) copolymer, initiated through an ionic mechanism. It is widely accepted that the cyclization of nitrile groups in PAN can be initiated only at a higher temperature
New precursors, poly(AN-CA) and poly(AN-IA-CA) copolymers, for the preparation of carbon fibers, were synthesized via a conventional free-radical polymerization. The synthesized PAN copolymers, which contain CA, showed higher