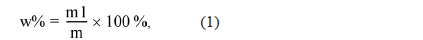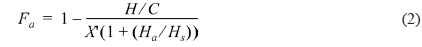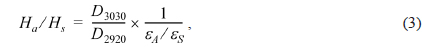Pitches are solid, fusible residues that are typically produced from coal, petroleum, and pure compounds [1]. Petroleum-based pitches are a low-priced raw material and are widely used in the manufacture of important industrial products for a wide range of carbon products, including carbon electrodes, high thermal conductivity carbon fibers, and the matrix phase of carbon–carbon composites [2-4]. Pitches with high carbon content can be prepared through the thermal polymerization of aromatic decant oil, a by-product of the fluid catalytic cracking of the heavy gas oil fraction. These pitches are complex materials and are usually composed of mixtures of polycyclic aromatic hydrocarbons, with an overall molecular weight ranging from approximately 200 to 2000 [5,6]. Reforming represents a series of reactions in which volatile components and gas are evaporated from raw materials and activated molecules are formed as generated free radicals that simultaneously pass through cyclization, aromatization, and poly-condensation. The properties of pitch are dependent on its chemical composition, which is determined by the heat treatment conditions used in its production. In the preparation of pitch, heat treatment of the petroleum or coal tar must be conducted at temperatures higher than 300°C for several hours [7,8]. This reforming process increases the cost due to the soaking time required [9,10]. Heat-soaking of pitches, which removes at least a portion of the aromatic oils present, results in high yields suitable for carbon artifact manufacture.
E-beam radiation has been widely applied in polymer modification, such as cross-linking, curing, and grafting because of the optical transparency of the system and the lack of requirement for a chemical/photochemical initiator [11]. Like other polymerization methods, with the generation of free radicals, E-beam-triggered polymerization reacts rapidly. The molecular weight of the polymer rapidly increases when free radical termination is minimized [12]. The reforming of petroleum-based precursor materials by E-beam radiation has not been reported.
In this study, we prepared a modified pitch from pyrolized fuel oil (PFO) by novel E-beam radiation. The effect of E-beam treatment on the elemental composition, chemical bonds, molecular weight, solubility, softening point, yield, and density of the prepared pitch was investigated. Based on these results, we evaluated the suitability of E-beam radiation as a method for obtaining pitches.
The pitch precursor used in this study was PFO, which was supplied by the GS Caltex Refinery Company in Korea. Aluminum chloride (AlCl3, 99%; Aldrich, USA) was used as a catalyst for reforming.
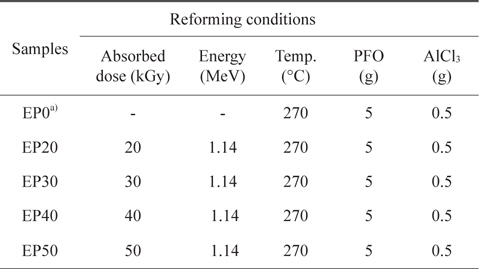
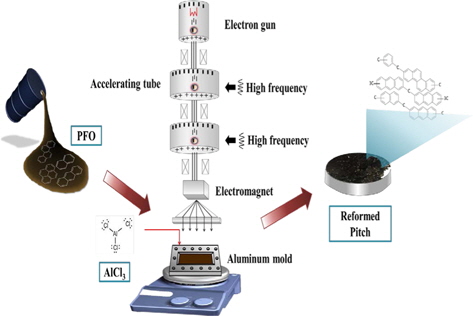
For the E-beam treatment, AlCl3-containing PFO were poured into an aluminum mold. The aluminum mold was sealed with a lid, placed on a hot plate, and heated at 270°C for 1 h. E-beam irradiation of the AlCl3-containing samples was subsequently conducted at 0, 20, 30, 40, and 50 kGy. As shown in Table 1, the prepared samples were labeled according to the absorbed doses as EP0, EP20, EP30, EP40, and EP50, respectively. The schematic procedure for reforming PFO is illustrated in Fig. 1.
[Table 1.] Pitch reforming conditions
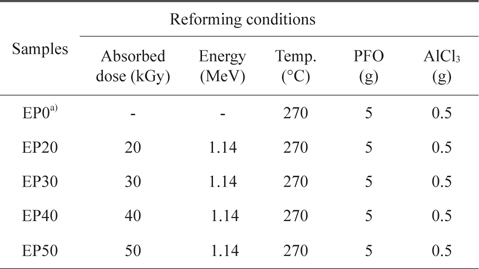
Pitch reforming conditions
Elemental analysis of the PFO and reformed pitch was performed using an elemental analyzer (EA1110; CE Instrument, Italy). Fourier-transform infrared spectroscopy (FT-IR; Bio-Rad Laboratories, Inc., FTS-175C) was used to analyze the chemical structure of the components in prepared pitches. The softening point and molecular weight distribution were investigated using a softening point apparatus (FP90; Mettler, Switzerland) and matrix-assisted laser desorption/ionizationtime of flight (MALDI-TOF; Bruker Daltonics, Germany), respectively. In addition, the toluene insoluble (TI) and quinoline insoluble (QI) content of the pitch was measured by the standard solvent extraction method. In a typical procedure, pitch was first dissolved in benzene or quinolone, and then the TI or QI component was collected from the suspension via filtration. The TI and QI contents were calculated as follows:
where w% is the percentage of TI or QI content, m1 is the weight of the TI or QI content, and m is the weight of the pitch.
3.1. Elemental properties of samples
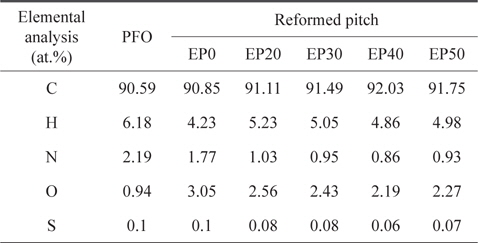
The elemental analysis results for PFO and reformed pitch are summarized in Table 2. The sulfur content of the PFO was very low (0.1 wt%) compared to commercial A-20 pitch (2.4 wt%), suggesting that PFO is an appropriate carbon material precursor [13]. The nitrogen, hydrogen, and sulfur contents of all pitches decreased slightly after heat and E-beam treatments. By contrast, the number of carbon elements in the reformed pitches increased as the absorbed dose of E-beam radiation increased, with the exception of EP50. This result indicates that the optimum condition for obtaining a higher carbon content and lower content of other elements is E-beam irradiation at 40 kGy. The EP0 sample did not solidify, demonstrating that only heat treatment without E-beam treatment was insufficient to obtain pitch.
[Table 2.] Elemental analysis of PFO and reformed pitches
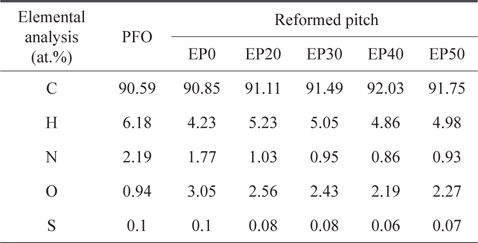
Elemental analysis of PFO and reformed pitches
3.2. Chemical analysis of samples
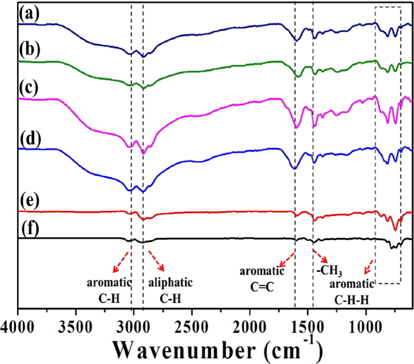
Fig. 2 presents the FT-IR spectra of PFO and the pitches reformed by different methods (E-beam and heat treatments), which exhibited adsorption bands associated with aliphatic bonds (C-H: 2920 cm-1) and aromatic bonds (C-H: 3037 cm-1, C=C: 1600 cm-1, C-H-H: 700-900 cm-1) [14,15]. The adsorption bands of aromatic bonds increased in response to heat and Ebeam treatments. This increase was clearly larger in E-beamtreated pitches compared to heat-treated samples, indicating that E-beam-treated pitches contained more aromatic carbon compounds than heat-treated pitches. Heat treated sample (EP0) did not solidify due to low contents of aromatic compounds. In addition, the adsorption bands of aromatic compounds increased more than those of aliphatic compounds with increasing absorbed doses. The increase in aromatic C-H and C=C bonds was particularly remarkable compared to other aromatic and aliphatic bonds. Also, the aromatic adsorption band was somewhat reduced for the high absorbed dose (EP50), indicating that the content of aromatic carbon compounds decreases when the absorbed dose is increased beyond a certain level.
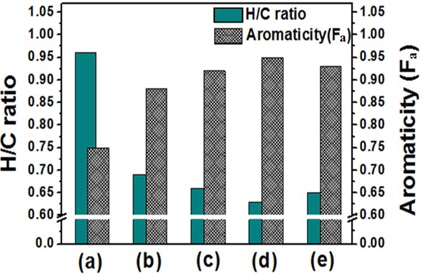
The atomic molar H/C ratio and the aromaticity (Fa) of PFO and the reformed pitches are shown in Fig. 3. The H/C ratio decreased from 0.96 for PFO to 0.63 for the EP40 sample. This drop is attributable to the conversion of oxygen and hydrogen to CO, CO2, and CH4 and the volatilization of these products during the E-beam treatment of high molecular weight components. Low molecular weight components were concurrently converted into aromatic compounds by poly-condensation [16]. The EP40 sample possessed the lowest H/C ratio. The Fa value was calculated from the spectra according to the following formula [17,18]:
where X’ is the average number of hydrogen atoms combined with non-aromatic carbon atoms, assumed to be 2; H/C is the atomic mole ratio;
3.3. Molecular weight distribution of the samples
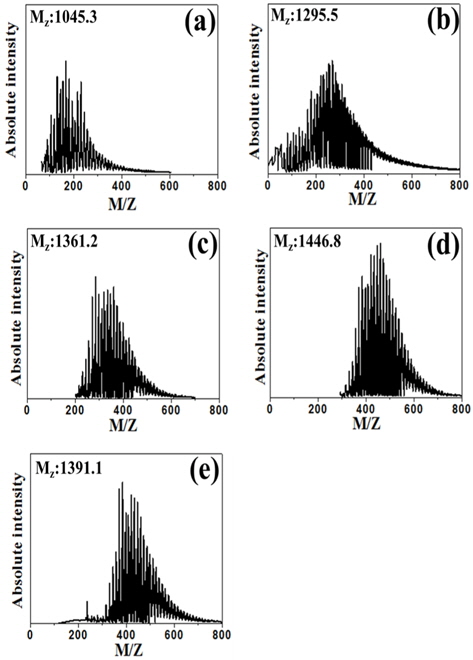
Fig. 4 shows the average molecular weight (Mz) of PFO and E-beam treated pitch, as calculated according to the following [19]:
where Ni is the molecular weight and Mi is the absolute intensity of the molecular weight. The Mz value of the samples increased from 1295.5 to 1391.1 in the order PFO < EP20 < EP30 < EP50 < EP40 with E-beam radiation treatment. This result confirms that low molecular weight components are converted to gas by E-beam radiation treatment, as indicated by the results of the elemental analysis. The formation of aromatic pitch by free radical polymerization is assumed to involve several elementary reaction steps, such as radical formation/initiation, free radical propagation, and the transfer of free radical activity to various molecules [20]. E-beam treatment at excessive absorbed doses resulted in a decrease in molecular weight, as confirmed in the EP40 and EP50 samples. As shown in the MALDI spectrum, the range of the molecular weight distribution became narrower following E-beam irradiation because low molecular weight components were converted to gas or polymerized with other molecules, while high molecular weight components were simultaneously decreased by E-beam irradiation. Thus, E-beam radiation treatment did not increase the molecular weight substantially. Molecular growth and the narrow molecular weight distribution indicate that aromatic compounds of similar molecular weight were formed in pitch by E-beam reforming.
3.4. Solubility of the samples
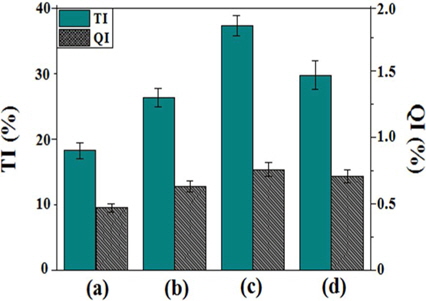
Fig. 5 shows the solubility results of reformed pitches obtained by various absorbed radiation doses. The TI and QI contents of the reformed pitches were 18.2%-29.7% and 0.47%-0.76%, respectively. The QI value was relatively low compared to that of coal tar pitch due to the lower number of aromatic rings per molecule [21]. Both the QI and TI values increased proportionally with increasing absorbed doses in the range of 20-40 kGy. At the molecular level, both E-beam and heat treatment led to a decrease in aliphatic side chains, while cyclization increased the amount of aromatic hydrogen and carbon. This behavior indicates the occurrence of dealkylation of the alkylaliphatic side chains combined with polymerization and condensation of the aromatic rings. However, the results of E-beam treatment differ from those of heat treatment alone, as confirmed in the EP50 sample and other samples. Generally, TI and QI increased proportionally with increasing soaking temperature [22]. However, for E-beam reforming, a high energy input to the petroleum-based precursor materials did not result in a continuous increase in the TI and QI fractions. This result indicates that E-beam treatment reforming follows a different process than heat treatment with high energy radiation. The material generated by ionization is composed of a radical and an ion. When ionizing radiation reaches the material, several processes occur, such as chain scission, oxidation-reduction, and crosslinking [23]. High-energy electron radiation can initiate radical polymerization initiated by radiolysis. At the beginning of radiation, aromatic compounds in PFO polymerize rapidly, but decomposition of the polymerized compounds subsequently occurs with prolonged radiation time or an absorbed dose. This decomposition mainly occurred at E-beam irradiation doses of greater than 50 kGy. Based on the TI and QI content, the composition of the pitches can be classified as α-resin (QI), β-resin (TI-quinoline soluble), or γ-resin (toluene soluble), which exhibit different properties [24]. A high α-resin content characterizes pitches with high molecular weight, indicating an increased softening point. By contrast, a higher β-resin value is associated with the improvement of pitch properties such as molecular weight distribution and aromaticity. In addition, increased γ-resin values indicate a decreased softening point due to the presence of low molecular weight compounds [25]. The β-resin value of EP40 is the highest due to the sharp increase in QI and slight increase in TI with an increased absorbed dose, as indicated by the difference in the heights of the bars corresponding to QI and TI. EP50 is the exception to this trend. Therefore, appropriate E-beam radiation can increase the β-resin content of reformed pitch.
3.5. Softening point of the reformed pitch
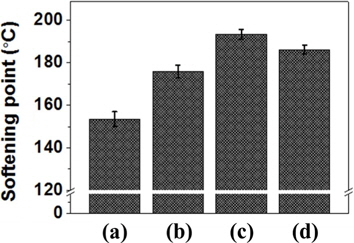
As shown in Fig. 6, the softening points of EP20, EP30, EP40, and EP50 were 153°C, 175°C, 193°C, and 186°C, respectively. These values followed a trend with the adsorbed dose of E-beam irradiation being similar to those for the molecular weight and insoluble fraction. In the range of 20-40 kGy, the softening point increased with an increased absorbed dose. However, the softening point of the EP50 sample was lower than that of the EP40 sample. This result indicates that appropriate E-beam radiation can increase the softening point and that organic compounds formed from PFO can decompose during reforming due to the powerful energy intensity of E-beam treatment [26]. With exposure to the electron beam, most of the growing chains will be terminated because of the high radical concentration created by radiation with a high dose rate. By contrast, polymer chains form and grow at lower electron beam doses. Thus, with an increased absorbed dose and longer irradiation time, more polymer chains of low molecular weight will be formed, leading to a decline in the molecular weight and softening point of the pitch.
3.6. Yield and density of the reformed pitches
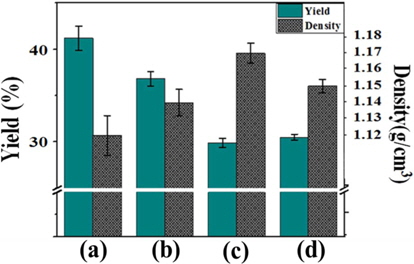
Fig. 7 shows the yield and density of the pitches as a function of the E-beam reforming conditions. The yield of reformed pitches decreased from 41.2% to 29.9% in the dose range of 20-40 kGy, in contrast to the trend of density. This result indicates that decomposition, cyclization, and dehydrogenation reactions actively occurred as reforming parameters, such as absorbed dose and radiation time, increased, which led to the production of volatilized gas. Compared with the other treatments, decomposition primarily occurred in the E-beam treatment at 50 kGy, resulting in a slightly increased yield of 30.5%. By contrast, the density of E-beam-treated pitches increased with increasing absorbed doses. This result indicates that densification progressed due to the removal of gas, which was converted from low molecular weight components by E-beam treatment [22].
In this study, pitch was reformed and prepared from PFO by a novel E-beam treatment, and the suitability of E-beam radiation as a reforming method was evaluated. The nitrogen, hydrogen, and sulfur contents of the pitches decreased after E-beam treatment, while the H/C ratio decreased because oxygen and hydrogen were converted to CO, CO2, and CH4, and were volatilized. The aromaticity (Fa) of the reformed pitch increased with E-beam treatment and reached its highest value at the highest appropriate absorbed dose (40 kGy), indicating the presence of more aromatic compounds. The range of the weight distribution became narrower because aromatic compounds were generated with similar molecular weights. Both the QI and TI values were proportionally increased with increasing absorbed doses in the range of 20-40 kGy. Above 50 kGy, the decomposition of compounds primarily occurred, resulting in a decrease in the insoluble fractions. The softening points and molecular weight of E-beam-treated pitch exhibited similar trends due to modification by free radical polymerization and the powerful energy intensity of E-beam treatment. In conclusion, the novel E-beam radiation reforming presented here is an effective method for preparing aromatic pitch with β-resin components.