In tasar silkworm
The neurosecretory cells consist of nucleus at the center and various cell organelles in the perikarya. The cell organelles include ribosomes, Golgi complexes and endoplasmic reticulum. All organelles are involved in the synthesis of neurosecretory material (NSM) in the form of granules (Nishiitsutsuji-uwo, 1960).
The ultrastructure of intracellular organization and subcellular mechanism associated with the synthesis and release of NSM by the MNC in the brain was studied earlier in some Lepidoptera (Ichikawa and Nishiitsutsuji-uwo, 1959, 1960; Ichikawa and Takahashi, 1959; Nishiitsutsuji-uwo, 1960). There is however, no information on the ultrastructure of MNC in tasar silkworms.
The present study was therefore, undertaken to study the ultrastructure of the MNC, secretion of neurosecretory granules (NSG) and release of NSM in the pars intercerebralis of brain in tasar silkworm
Tasar silkworm,
>
Ultrastructure of median neurosecretory cells
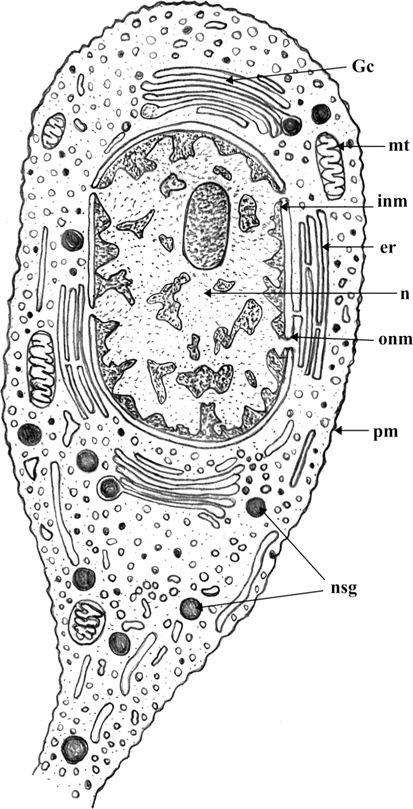
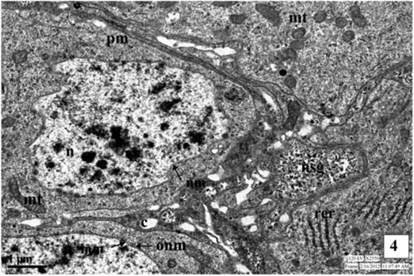
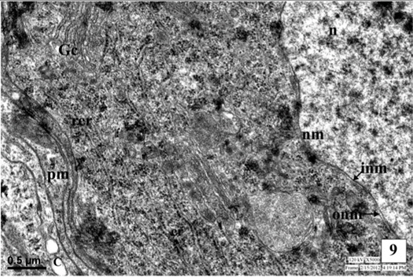
The medial neurosecretory cells are spherical or oval in shape. The plasma membrane is well differentiated and some canaliculies are associated with the plasma membrane. The electron microscopic studies show the presence of nucleus at the center of the cells. Most of the region of the perikaryon is filled with concentric long granular endoplasmic reticulum (GER), mitochondria (MT), Golgi complexes (GC) in the close vicinity to NSG (Fig. 1). The mitochondria are rod shaped. The NSG are often accumulated throughout the cytoplasm and axonal endings.
Nucleus (N)
Nucleus is situated at the center of the cell, spherical or oval in shape and contains dispersed chromatin material and prominent nucleoli of irregular shape. The electron micrographic study reveals the nuclear membrane (NM) consisting of two layers, the inner thick and outer thin layers. Sometimes, the outer layer is associated with the endoplasmic reticulum.
The nucleus contains many dense granules, evenly distributed or aggregated into small colloids. The nucleolus appears as a dark area with irregular shape (Fig. 1).
Cytoplasmic organelles and neurosecretion
The cytoplasm of neurosecretory cells in the pars intercerebralis is well equipped with the cytoplasmic is organelles viz., mitochondria, endoplasmic reticulum, Golgi complex and lysosomes. The cytoplasm also filled with electron-dense as neurosecretory granules (NSG) (Fig. 1).
Mitochondria (MT)
In the cytoplasm of MNC, numerous filamentous, rod like or sometimes beaded like mitochondria occur. They stain densely. The mitochondria are distributed at random or sometimes gather together in aggregates. Mitochondria consist of double membranes and cristae.
Endoplasmic reticulum (ER)
The endoplasmic reticulum (ER) is randomly scattered through the cytoplasm of the neurosecretory cells (Fig. 1). Most of the membranes of ER are rough-surfaced, interchanged with a few smooth-surfaced (SER) reticulae. The ribosomes were associated with the rough endoplasmic reticulum (RER).
Golgi complex (GC)
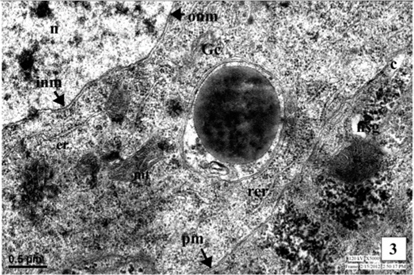
The Golgi complex is composed of stacks of smooth membrane and vesicles. The Golgi lamellae sometimes contain NSG at their terminal ends while, an electron dense material has been seen in the tubules and vesicles indicating involvement of Golgi complex in the synthesis of NSG. The NSG show gradual increase in their size after being budded off from the Golgi complex. The fully formed NSG are bounded by a membrane (Fig. 1).
Neurosecretory Granules (NSG)
The neurosecretory granules are observed in the cytoplasmic perikarya of NSC as the fine, spherical or ellipsoidal, electrondense elementary granules (EG) (Fig. 1). The granules were relatively uniform in electron density, and they measure about 500-2500Å (1500Å in diameter). The NSG are often observed in the axonal endings of the neurosecretory cells.
Synthesis of the NSM
The MNC show synthesis of fine, spherical or ellipsoidal, electron-dense NSG. The NSG are formed in close vicinity of the mitochondria, Golgi zone and rough endoplasmic reticulum. The NSG are frequently encountered near the Golgi zone.
The NSG are scanty and are enveloped with membranous covering. The NSG are evenly distributed in the perikarya of NSC.
Release and transport of NSM
The NSG are migrated along the axons of neurosecretory cells of the brain to the corpora cardiaca via neurosecretory pathways. The direct release of neurosecretory material appears from the perikarya of neurosecretory cells into the canaliculi, communicated to the blood sinus. The canaliculi are closely associated with the plasma membrane of NSC. The NSM is directly released from cell into the haemolymph through the process of exocytosis.
>
Ultrastructure of MNC during larval development
Ultrastructure of NSC in I instar
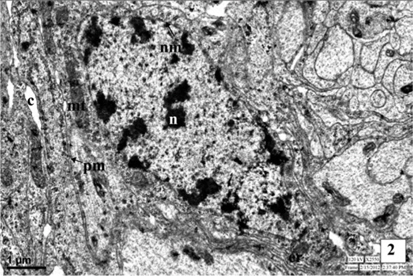
The neurosecretory cells in the first instar larva are observed in the pars intercerebralis of brain. The nuclei are situated at the center of the cell and measures about 5.5μm. Nuclei are oval in shape with irregular outline, the nuclear membrane is double layered. The perikarya of NSC contain numerous mitochondria, endoplasmic reticulum and Golgi apparatus. The cell organelles are closely associated with formation of NSM in the form of NSG. The small electron dense granules are observed in the cytoplasm as well as axonal endings of the MNC. The NSG measure about 472 Å in diameter. Some canaliculi are also associated with the plasma membrane of MNC (Fig. 2-3 and Fig. 13).
Ultrastructure of NSC of II instar
The spherical nucleus is placed centrally, measured about 4.25 μm to 8.75 μm. The nuclear membrane consists of two layers, inner 44.25 nm and outer 23 nm in thickness. The outer layer of nucleus is associated with the endoplasmic reticulum. The mitochondria are distributed throughout the perikarya of the neurosecretory cells. The mitochondria in number in the cell as compared to the first instar larva. Golgi complexes are also appeared in the cytoplasm. The canaliculi are associated with the plasma membrane of NSC for the direct release of NSM in to the blood sinus.
The NSG are closely associated with ER and MT. The size of NSG increases up to 776 Å. The amount of NSG increases in quantity in the cytoplasm of NSC, neurosecretory pathways and axonal endings (Fig. 4-5 and Fig. 13).
Ultrastructure of NSC of III instar
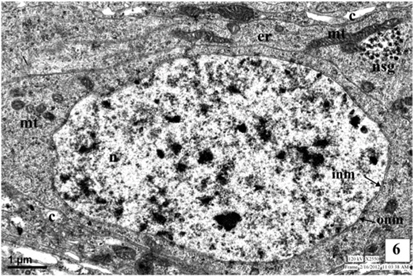
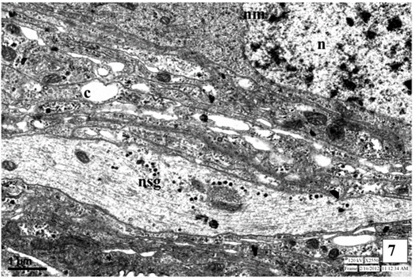
The ultrastructure of neurosecretory cells in the third instar larva is similar to the I and II instars. The spherical big sized nuclei with double layered nuclear membrane (NM) are observed at centre of the cell. The increased number of cell organelles like, mitochondria, Golgi bodies and endoplasmic reticulum is well evident in the perikarya. The organelles are observed in close vicinity with each other and are involved in the synthesis of NSG. The plasma membrane of NSC is attached with the canaliculi.
The NSG are well evident in the cytoplasm and the axonal endings and neurosecretory pathways of the NSC. The NSG measures about 1245Å in diameter (Fig. 6-7 and Fig. 13).
Ultrastructure of NSC of IV instar
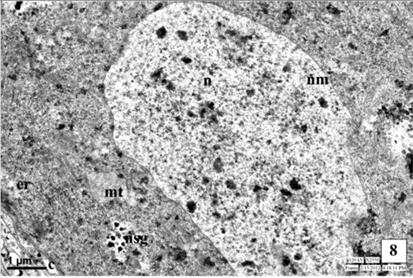
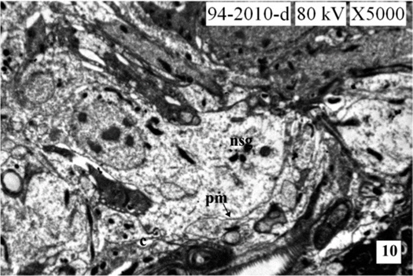
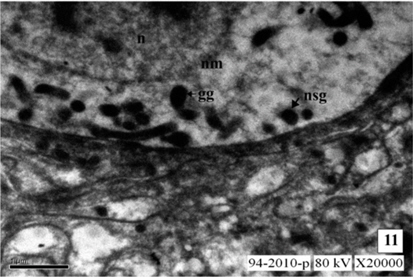
The neurosecretory cells in the pars intercerebralis of fourth instar larva are similar to the cells of III instar larva. The mitochondria (MT) are distributed in the cytoplasm of the cells. The Golgi bodies and the channels of rough endoplasmic reticulum (RER) are well evident. They are closely associated with each other. The interrelationship between the organelles viz., MT, ER and Golgi bodies for the synthesis of NSM is well evident. The cell organelles increase in number indicated the excess secretion of NSM than in the third instar. The size of NSG increases than that in the third instar and measure about 1622Å in diameter. The NSG are observed in the perikarya, axonal endings of NSC and neurosecretory pathways of NSC (Fig. 8-9 and Fig. 13).
Ultrastructure of NSC of V instar
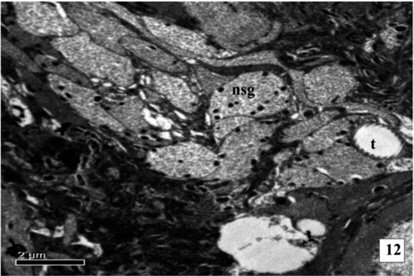
The NSC contains well developed nuclei enveloped by double layer nuclear membrane. Cell organelles are well developed and abundant in number. The endoplasmic reticulum (ER), Golgi complex (GC) and mitochondria (MT) are well distinguished and are closely associated with the synthesis of NSM. The NSG are observed in the Golgi zone. The NSG are transported by the axons as well as the canaliculi to the corpora cardiaca (CC) and blood sinus. The neurosecretory pathways are distinctly observed. The synthesis of NSM is increased and the NSG are distributed in the perikarya and transported through axons. The NSG appeared spherical and dark black in colour. The NSG are increase in size and large sized granules are found in cytoplasm. The synthesis of NSM increases and the cells are full of NSG. The NSG are of two types, giant granules (GG) measuring about 7025 Å and smaller elementary granules (EG) measuring about 2275Å in diameter.
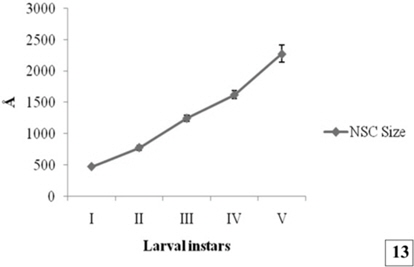
During larval development the secretory activity by the NSC are observed predominantly. The NSG are observed frequently in the Golgi zone, cytoplasm, neurosecretory pathways and axonal endings of cells. The size of NSG increases gradually from the first to the fifth instars larva (Fig. 10-12 and Fig. 13).
The electron microscopic study of the neurosecretory cells of tasar silkworm
The MNC in the pars intercerebralis of the larvae of
On the basis of the dimension,
Scharrer and Scharrer (1944) noticed the neurosecretory granules in the neurosecretory cells of
The electron micrographs indicate that the neurosecretory cells in the pars intercerebralis are enmeshed by the neuroglial cell processes, among which there exist canaliculi communicating with a blood sinus. The canaliculi are in contact with plasma membrane of the neurosecretory cells. Although elementary granules cannot be seen within the canaliculi, these structures are apparently convenient for direct secretion of the secretory material from cells into blood system of
From the above observations, it may be summarized that the formation of the elementary granules occurres in the close association within the Golgi zone and it is a continuous process from the I to V instar larvae.