Tubificid oligochaetes are common and frequently dominant in freshwater benthic habitats. They are so tolerant to water pollution that they are often used as biological indicators. Faunistic studies of Korean freshwater oligochaetes have been actively conducted recently. The most well studied oligochaete family in Korea is the tubificids following the naidids. Nine species of tubificids have been reported so far. Nevertheless, many species of tubificids still remain to be discovered in Korea. In this study, we added four species of tubificid oligochaetes to the Korean fauna, including Linmodrilus profundicola (Verrill, 1871), Potamothrix heuscheri (Bretscher, 1900), Tubifex blanchardi Vejdovský, 1891, and Ilyodrilus templetoni (Southern, 1909) based on specimens collected from three locations in Korea: Cheonan-si, Geoje-si, and Seocheon-gun. In particular, P. heuscheri was first reported in Asia.
Many species of Oligochaeta occur in a variety of freshwater environments such as puddles, rice paddies, reservoirs, brooks, rivers, and streams. About 1,700 species are known (Caramelo and Martínez-Ansemil, 2012). Oligochaetes are small-sized worms, ranging from 1 mm to a few centimeters in length. Tubificids are a dominant group found in freshwater benthic habitats among aquatic oligochaetes, and consequently bear ecological importance. Their bodies are red, and their reproductive organs are well-developed during maturity. When alive, their head is in the bottom sediment, usually gregarious in habitat and most commonly found in soft sediments covered with organic matter (Schenková and Helešic, 2006). Oligochaetes serve as an important source of food for fish and other aquatic animals as well as decomposers. Some oligochaetes have been used to monitor water pollution in rivers and streams (Lin and Yo, 2008). As a result of recent faunistic studies (Park et al., 2013a, 2013b), nine species of tubificids have been described in Korea, including
Specimens were collected from March−May 2013 from Gyochon-ri, Mokcheon-eup, Dongnam-gu, Cheonan-si, Chungcheongnam- do; Sinseong-ri, Hansan-myeon, Seocheon-gun, Chungcheongnam-do; Siljeon-ri, Hacheong-myeon, Geojesi, Gyeongsangnam-do, Korea. Aquatic oligochate samples were collected using a hand shovel at the edge of a stream covered with fine sand or sediment rich in organic material. In the laboratory, the samples were sorted under a stereomicroscope while the organisms were alive. They were fixed in 5% formaldehyde or 70% ethanol solutions, and the specimens were stained in alcoholic paracamine and mounted whole in Canada balsam following the protocol of Erséus (1994). Identification and measurements were performed using slide-mounted specimens. Figures and pictures were made with a BX41 research microscope (Olympus, Tokyo, Japan) attached to a 650D digital camera (Canon, Tokyo, Japan). Measurements were made with an eyepiece micrometer or by pictures taken and analyzed with InnerViewTM- i series image analyzing software (Innerview Co. Ltd., Seongnam, Korea). The figures were drawn with a microscope drawing tube. These mounted collections are stored in the Lab of Ecology Genetic (LEG), Department of Science Education, Ewha Womans University. Other materials, preserved in 70% ethanol, were submitted to the National Institute of Biological Resources (NIBR) of the Republic of Korea.
Phylum AnnelidaClass ClitellataOrder HaplotaxidaSuborder TubificinaFamily Tubificidae Vejdovský, 1884Genus Limnodrilus Claparède, 1862
Key to the Korea species of the Genus
1. Thick walled cuticular architomy and sheaths cylindrical, usually much longer than broad, surrounded by spiral muscles ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙22. Penial sheath thin-walled, 50-80 times wider than sheath width ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙L. claparedeianus Penial sheaths up to 7 times longer than broad, mwith hood reflected back over shaft unless forced forward ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙L. profundicola* Length of penial sheath 4 times wider than sheath width ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙L. udekemianus
* Species of present study.
Tubifex profundicola Verrill, 1871: 451.Tubifex profundicola Verrill. Smith, 1874: 699.vcLimnodrilus alpestris Eisen, 1879: 10.Limnodrilus monticola Eisen, 1879: 18.Limnodrilus alpestris Eisen, Vejdovsky, 1884: 45; Eisen, 1886: 896, Pl. II, fig. 11a-h; PI. XVII, fig. 11i-k; PI. XIX, fig. 18; Beddard, 1895: 254; Rybka, 1898: 390; Michaelsen, 1900: 44; 1914: 16, PI. V, fig. 5; Galloway, 1911: 315.Limodrilus monticola Eisen, Vejdovsky, 1884: 45; Eisen, 1886: 896, Pl. XI, fig. 10a-h; Beddard, 1895: 254; Michaelsen, 1900: 46; Galloway, 1911: 315.Clitellio (Limodrilus) alpestris (Eisen). Vaillant, 1890: 428.Clitellio (Limodrilus) monticola (Eisen). Vaillant, 1890: 427.Linmodrilus helceticus Piguet, 1913: 134, figs. 8-10.Limnodrilus helveticus Piguet. Piguet and Bretsher, 1913: 79, fig. 19b; Hrabě, 1954: 306; Juget, 1957: 3; 1958: 90, fig. 14a, b; Malevitch, 1957: 82; Sokolskaya, 1958: 310; 1961a: 56; 1961b: 85; Brinkhurst and Kennedy, 1962: 185; Moszynska, 1962: 27; Brinkhurst, 1963: 38, fig. 24a-e, 41, fig. 12g.Limnodrilus profundicola (Verrill). Brinkhurst, 1965: 130, fig. 4k-m.
Material examined. All specimens were collected from Gyochon-ri, Mokcheon-eup, Dongnam-gu, Cheonan-si, Chungcheongnam-do, Korea, 36˚51′38.93′′N, 126˚38′27.29′′E, 21 May 2013 (collector Lee J). Small and shallow pools with the bottom covered in sludge such as soft mud and organic matter. CBCA 1305211: mature, mounted on a slide, deposited at LEG. KOSPIV0000193713: mature, preserved in 70% ethanol solution, deposited at NIBR.
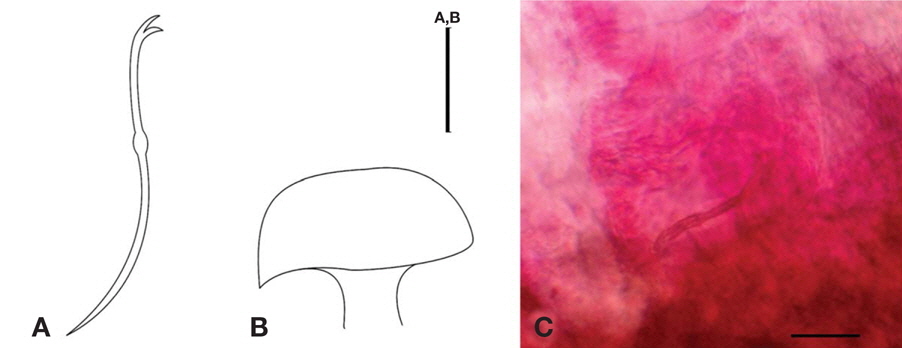
Diagnosis. Length 13.85 mm, width at X 0.76 mm, number of segments 87.
No eye present. Ventral chaetae of anterior segments, bifid, arranged in two rows of 6-8 per bundle, with teeth of equal length (Fig. 1A). Dorsal chaetae with no hair chaeta, similar form to ventral chaetae. Penial sheaths in segment X, up to 7 times longer than broad, with head plate reflected back over the shaft, representing an umbrella or mushroom shape (Fig. 1B, C). Dark chloragogen tissue on oesophagus beginning from VI.
Distribution. Holarctic.
Remarks.
Tubifex heuscheri Bretscher, 1900: 11, PI. I, figs. 1-4.Tubifex heuscheri Bretscher. Bretscher, 1905: 664; Piguet, 1906: 391; 1913: 127, fig. 4a, b.Ilyodrilus heuscheri (Bretscher). Piguet, 1913: 127; Stammer, 1932: 578; Jaroschenko, 1948: 57; Hrabě, 1950: 280; Juget, 1958: 89, fig. 141; Cekanovskaya, 1962: 260, fig. 159.Tubifex (Ilyodrilus) heuscheri Bretscher. Piguet and Bretscher, 1913: 69, figs. 14a, 7b.Ilyodrilus orientalis Cernosvitov, 1938: 545, figs. 16-23.Ilyodrilus orientalis Cernosvitov. Hrabě, 1950: 279.Euilyodrilus orientalis (Cernosvitov). Brinkhurst, 1963: 51.Euilyodrilus orientalis (Bretscher). Brinkhurst and Kennedy, 1962: 184; Brinkhurst, 1963: 49, fig. 34.
Material examined. All specimens were collected from Siljeon- ri, Hacheong-myeon, Geoje-si, Gyeongsangnam-do, Korea, 34˚58′27.35′′N, 128˚39′23.65′′E, 25 Apr 2013 (collector Lee J). The sampling location was an agricultural waterway and small brook. The location was polluted with sewage and organic matter. GSGJ1304251: mature, mounted on a slide, deposited at LEG. KOSPIV0000193717: mature, preserved in 70% ethanol, and deposited at NIBR.
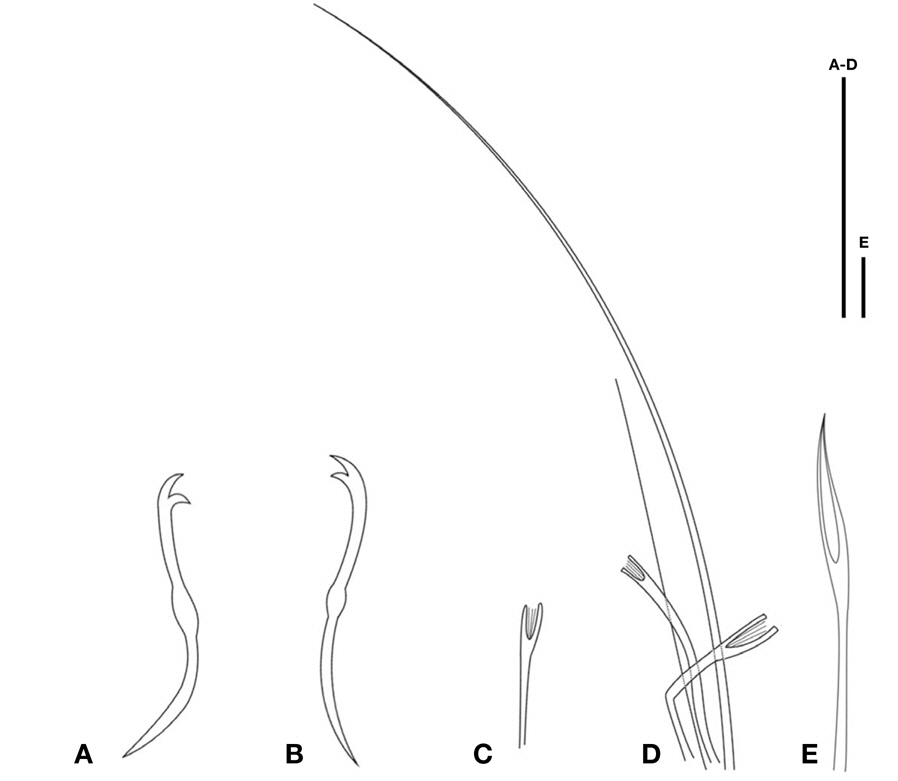
Diagnosis. Length 8.27 mm, width at X 0.29 mm, number of segments 64.
Prostomium short conical and separated by a distinct furrow. Ventral chaetae in II, 2-4 per bundle, with upper tooth straight, longer than the lower (Fig. 2A, B). Hair chaeta 1-2 per bundle, with pectinate chaetae, with relatively long and straight teeth and several slightly shorter intermediate denticles (Fig. 2C, D). Spermathecal chaetae mostly with narrow, hollow-tipped with parallel edges of the distal grooved portion, and with hooked distal end (Fig. 2E). Chloragogen cells from II onwards.
Distribution. Western Palaearctic, East Africa, South America.
Remarks. This specimen had spermathecal chaetae that have a long needle with slightly curved tip, which is characteristic of
Genus
Key to the Korean species of the Genus
1. Prostates attached to anterior face of upright atria with broad apically and narrow gradually towards the penial bulbs, vasa deferentia frequently very long ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙2 2. Chaeta bundle containing all chaeta types, muscular penial bulb present ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙T. tubifex Chaetae are comparatively straight and thin in both anterior and posterior crotchets, penial with truncated conical, thick-walled. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙T. blanchardi*
* Species of present study.
Tubifex tubifex f. Blanhardi Vejdovsky, 1891
Material examined. The specimen was collected from Sinseong-ri, Hansan-myeon, Seocheon-gun, Chungcheongnam-do, Korea, 36˚4′6.54′′N, 126˚51′42.94′′E, 22 Mar 2013 (collector Lee J). The sampling site was a shallow ditch of rice paddy with soft sediments at the bottom. CNSC130322a1: mature, mounted on a slide, deposited at LEG.
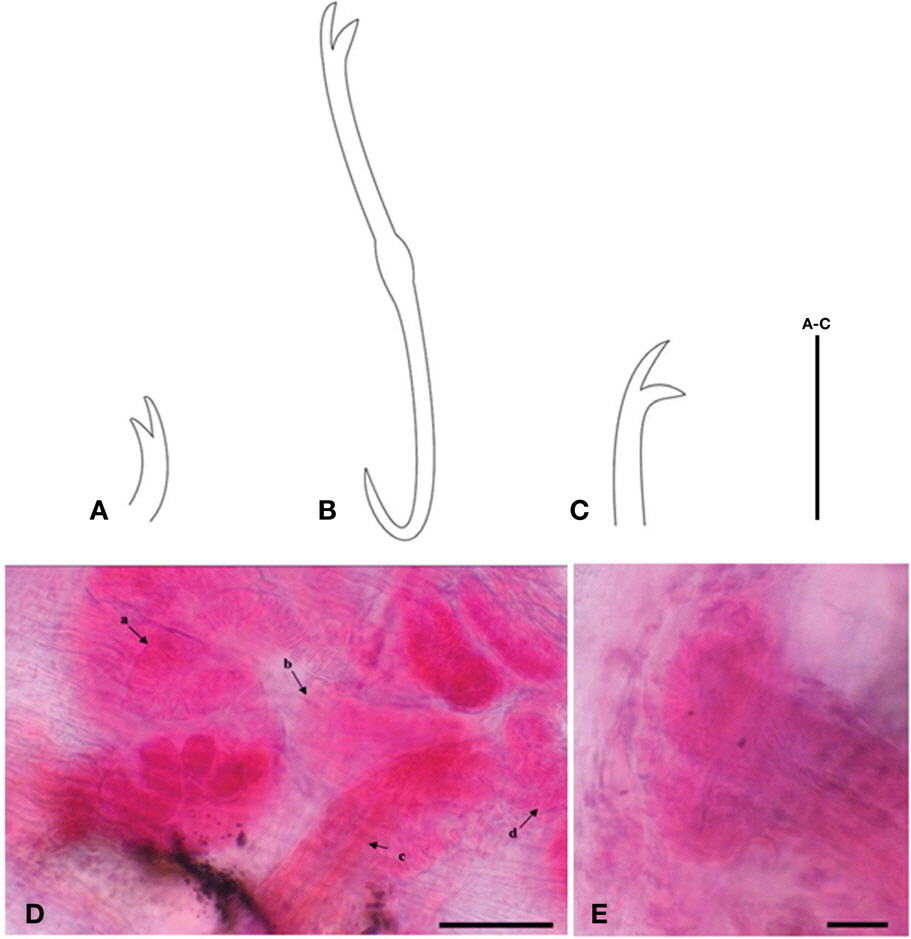
Diagnosis. Length 20.19 mm, width at XI 0.61 mm, number of segments 78.
Ventral chaetae in II, 5-6 per bundle, with slightly longer upper tooth in anterior segments (Fig. 3A). Hair chaetae absent. Clitellum is muff-shaped and extends from mid segment X to the end of segment XII. Sexual organs in XI. Vasa deferentia coiled ducts, thin-walled inside, thick-walled outside (Fig. 3B). Penial with truncated conical, thick-walled inside with glandular cells (Fig. 3C).
Distribution. Europe, North Africa.
Remarks.
Tubifex templetoni Southern, 1909: 140, Pl. VIII, fig. 6a-e; Friend, 1912: 292; Lastockin, 1927: 67; Brinkhurst, 1962: 326, fig. 1n; 1963: 23; 1965: 124, fig. 2e-g; Cekanovskaya, 1962: 275; Hrabě, 1962: 308; Kennedy, 1964: 228.Tubifex templetoni var typical Brinkhurst, 1963: 37, fig. 9d.Tubifex templetoni var walshi Brinkhurst, 1963: 42, fig. 9c; 1966: 736, fig. 1j-1.Ilyodrilus temletoni (Southern). Hrabě, 1966: 61, figs. 9-20.
Material examined. All specimens were collected from Sinseong-ri, Hansan-myeon, Seocheon-gun, Chungcheongnam-do, Korea, 36˚4′6.54′′N, 126˚51′42.94′′E, 22 Mar 2013 (collector Lee J). The sampling site was a narrow ditch of rice paddy with soft sediments at the bottom. CNSC130322b1, CNSC130322b2: immature (we observed sexual organs as formation of sexual organs was in progress), mounted on slides, deposited at LEG. KOSPIV0000193718: immature, preserved in 70% ethanol, deposited at NIBR.
Diagnosis. Length 6.99-15.70 mm, width at XI 0.36-0.42 mm, number of segments 102-121.
Ventral chaetae with the upper tooth slightly longer and thinner than the recurved lower tooth with no intermediate tooth, 2−5 per bundle (Fig. 4A−C). Vasa deferentia as long as the atrium. Chitinous penial sheath long, conical in their expanded proximal end in X, disto-lateral opening (Fig. 4D, E). Penial chaetae absent, no spermatozeugmata.
Distribution. Europe, North America, Asia, South Africa.
Remarks.