The cytochrome P450 (CYP) subfamily is one of the most important groups for biotransformation of xenobiotics and endogenous compounds.1 The regulation of drug-metabolizing enzymes is a major cause of numerous drug-drug and herb-drug interactions.2 CYP modulation is of considerable clinical importance and is known to occur through both enzyme induction and direct inhibition. Therefore, various assays have been developed to determine CYP activities in CYP enzyme sources such as liver microsome, recombinant enzyme, and hepatocyte.3 Over the last decade,
Licorice roots of the
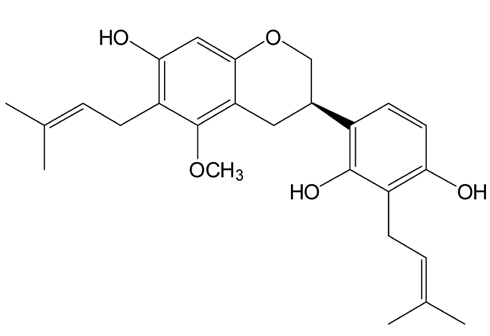
The licoricidin used in this study was isolated from the roots of
>
Inhibition of CYP2B6 and CYP2C9 in HLMs by licoricidin
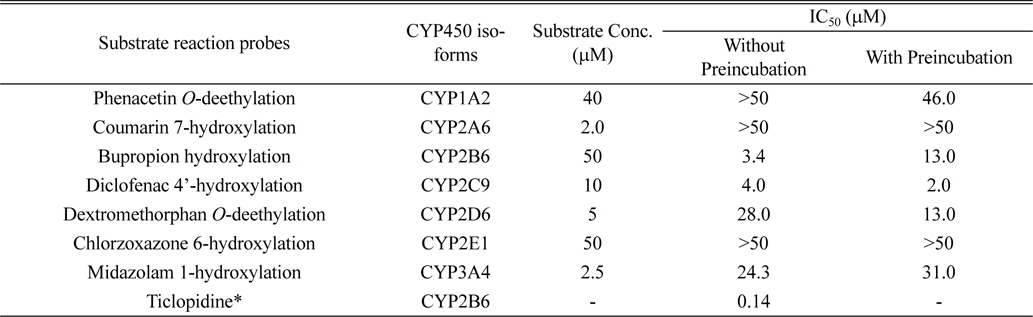
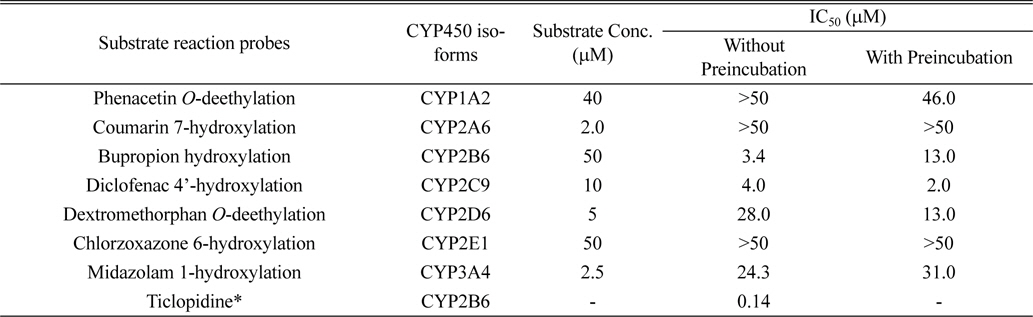
The inhibitory effects of licoricidin on the metabolism of the following seven CYP-specific substrates were examined: 2 μM phenacetin for CYP1A2, 50 μM coumarin for CYP2A6, 10 μM bupropion for CYP2B6, 10 μM diclofenac for CYP2C9, 5 μM dextromethorphan for CYP2D6, 50 μM chlorzoxazone for CYP2E1, and 2.5 μM midazolam for CYP3A.3,10 All incubations were performed in duplicate, and the data are presented as means. To investigate the inhibitory effect of licoricidin on the activity of seven CYPs, each reaction was performed with 0.5 mg/mL pooled HLMs in a final incubation volume of 0.1 mL. The incubation mixture contained 0.1M potassium phosphate buffer (pH 7.4), cocktail probe substrates, licoricidin, and an NADPH-generating system (NGS) containing 0.1M glucose 6-phosphate, 10 mg/mL β-NADP+, and 1.0 U/mL glucose-6-phosphate dehydrogenase. A mixture with licoricidin at final concentrations of 0-25 μM was prepared by adding NGS for 60 min without preincubation, or with preincubation for 5 min without cocktail probe substrates. After incubation, the reaction was stopped by adding 100 μL of acetonitrile containing 0.1% formic acid and 5 μL of internal standard solution (10 μM reserpine) in methanol. After mixing and centrifugation at 13,000 g for 10 min, a 10-μL aliquot was injected onto a C18 column for LC-MS/MS analysis. Furthermore, to characterize the mode of inhibition of CYP2B6 and 2C9 by licoricidin, 0.5 mg/mL HLMs were incubated with licoricidin at 0-8 μM in 0.1 M potassium phosphate buffer (pH 7.4) for 60 min at 37℃. Bupropion was used at a probe substrate at 25, 50, and 100 μM, and the corresponding values for diclofenac were 5, 10, and 20 μM.
>
Inactivation of human recombinant cDNA-expressed CYP2B6, and CYP2C9 by licoricidin
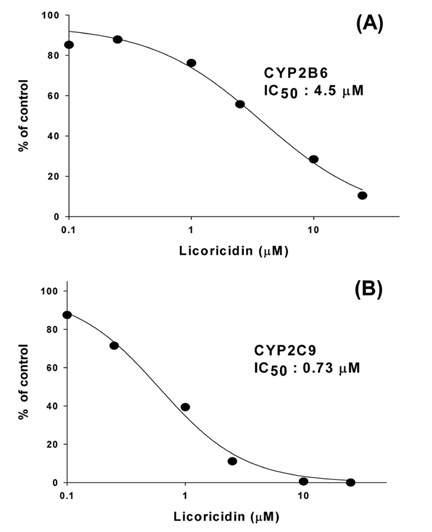
To confirm the selective inhibition of CYP2B6 and 2C9 isoforms by licoricidin, 10 pmol of human recombinant cDNA-expressed CYP2B6 or 2C9 was incubated with 0.1-25 μM licoricidin and NGS for 60 min at 37℃ after the addition of 50 μM bupropion and 10 μM diclofenac as selective CYP2B6 and 2C9 substrates, respectively.
LC-MS/MS assays were performed in the multiple reaction monitoring mode (MRM), and an AccelaTM LC system coupled to a TSQ Vantage triple quadrupole mass spectrometer (Thermo Fisher Scientific Inc., USA) equipped with a HESI-II Spray source was used. Electrospray ionization was performed in the positive mode at a spray voltage of 3,500 V (except for the detection of hydroxyl chlorzoxazone). Nitrogen was used as a sheath and auxiliary gas at optimum values of 45 and 20 (arbitrary units), respectively. Vaporizer and capillary temperatures were 150 and 300℃, respectively. For LC analysis, an Inertsil® ODS-2 column (3 μm, 2.1 × 150 mm, GL science) was used. The mobile phases consisted of LC-grade water containing 0.1% formic acid (A) and LC-grade acetonitrile containing 0.1% formic acid (B). The initial composition was increased to 95% solvent (B) over 10 min. A gradient program was used for HPLC at a flow rate of 220 μL/min. Multiple reaction monitoring was used for the detection of the CYP isozyme-specific marker metabolites. The precursor-product ion pairs used for monitoring the metabolites generated were as follows:
All incubations were performed in duplicate, and data are presented as means. Half maximal inhibitory concentration (IC50) values were obtained using percent activity versus log[I] concentration plots. Kinetic parameters were estimated by curve fitting using SigmaPlot (version 12.0, Systat Software, Inc.).
In this study, we used the cocktail probe to determine the activity of seven CYPs simultaneously; phenacetin for CYP1A2, coumarin for CYP2A6, bupropion for CYP2B6, diclofenac for CYP2C9, dextromethorphan for CYP2D6, chlorzoxazone for CYP2E1, and midazolam for CYP3A4 (Table 1). LC-MS/MS system in MRM mode was optimized for the detection for each metabolite. When licoricidin was incubated at 0-25 μM with CYP probes for 60 min at 37℃, licoricidin showed potent inhibitory effects on CYP2B6-catalyzed bupropion hydroxylation and CYP2C9-catalyzed diclofenac 4’-hydroxylation with IC50 values of 3.4 and 4.0 μM, respectively (Table 1). The IC50 values of licoricidin for CYP2D6 and 3A4 activities were higher than 20 μM, indicating a weak inhibitory effect. Other CYPs, including CYP1A2, 2A6, and 2E1, were not significantly inhibited by licoricidin. When the metabolic stability of licoricidin was evaluated in HLMs in the presence of NGS, the initial concentration of licoricidin diminished by less than 10% after incubation for 90 min, suggesting that licoricidin is metabolically stable and not a suicide inhibitor of CYP2B6 and 2C9.
Inhibitory effects of licoricidin on the activities of hepatic CYPs in human liver microsomes
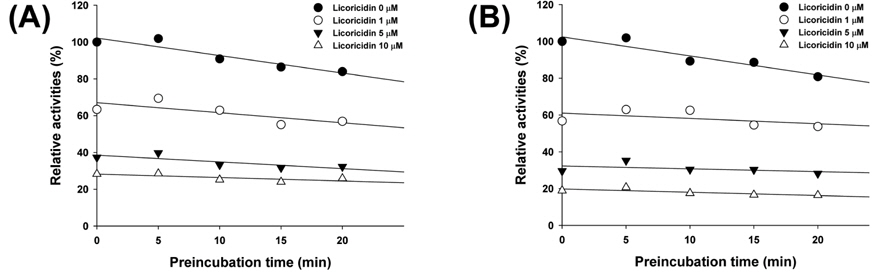
To investigate the mechanism underlying licoricidin’s inhibition of CYP2B6-catalyzed bupropion hydroxylation and CYP2C9-catalyzed diclofenac 4’-hydroxylation, the inhibitory activities (IC50 values) were determined both with and without pre-incubating microsomal incubation mixtures for 15 min at 37℃ in HLMs. The IC50 value of CYP2B6-catalyzed bupropion hydroxylation after preincubation was increased and it of CYP2C9-catalyzed diclofenac 4’-hydroxylation was not changed by preincubation. The pattern of IC50 shift showed the typical competitive inhibition. In addition, Figure 2 shows strong and dose-dependent inhibition, but not time-dependent inhibition, by licoricidin in HLMs.
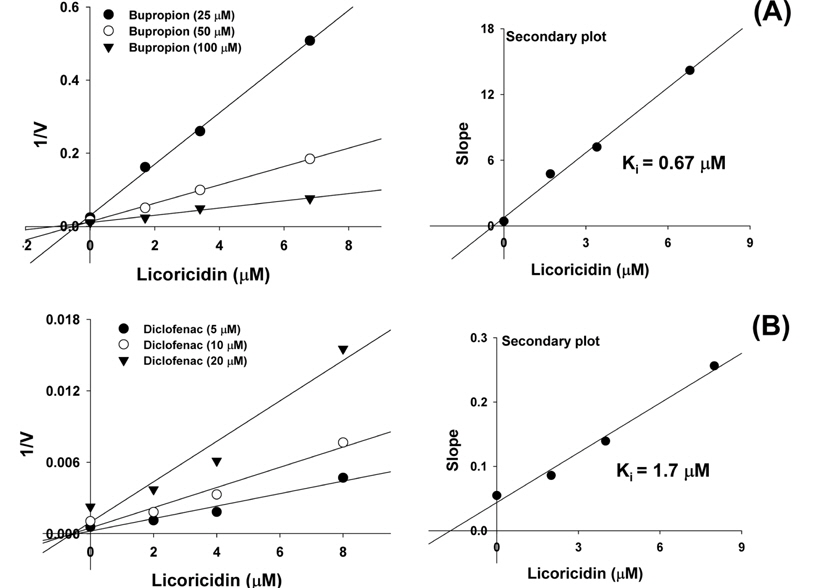
To investigate the mode of CYP2B6 and CYP2C9 inhibition by licoricidin in HLMs, Lineweaver-Burk plots were constructed by kinetic study of CYP2B6-catalyzed bupropion hydroxylation and CYP2C9-catalyzed diclofenac 4’-hydroxylation in the presence of 0-6.8 and 0-8 μM licoricidin, respectively. The Lineweaver-Burk plots collected on the y-axis and secondary plots were linear (Figures. 3A and B) indicating competitive inhibition of licoricidin with a
Identification of a potent inhibitory effect of an herb compound is of importance for investigating potential herbdrug interaction. CYP2B6 has been estimated to represent approximately 1-10% of the total hepatic CYP content and metabolize approximately 8% of clinically used drugs (n > 60) and endogenous materials.11,12 CYP2B6 is one of the CYP enzymes that bioactivates several procarcinogens and toxicants.1 CYP2C9 is one of the most important CYP enzymes involved in approximately 20% of CYP-mediated drug metabolism.13 Specifically, the substrate of CYP2C9 is clinically important to drugs with a narrow therapeutic index, such as warfarin and phenytoin.14 Therefore, the accidental regulation of CYP2C9 activity could lead to severe toxicity. Furthermore, the potent inhibitory effect of licoricidin on CYP2B6 and CYP2C9 activity could be responsible for blocking CYP-related carcinogenesis and/or producing potential herb-drug interactions with substrates that mainly undergo CYP2B-and CYP2C9-mediated metabolism.
In this study, the selective and potent inhibitory effects of licoricidin on CYP2B6 and 2C9 activity in HLMs were determined using a cocktail assay coupled with an LCMRM strategy. CYP2B6 and CYP2C9 are the most important enzymes accounting for approximately 30% of CYP-originated drug metabolism. Therefore, the administration of herbal products including licoricidin or licorice roots could cause a toxic herb-drug interaction with CYP2B6 and CYP2C9 substrate drugs. A clinical study is recommended to further investigate the potential for interaction.