Dutasteride, a competitive and selective inhibitor of both type I and II 5-α-reductases, is used for the treatment of benign prostatic hyperplasia and hair loss.1-6 While its potent pharmacological activity makes it popular in clinical fields, its formulation in market is only limited to a soft gelatin capsule filled with dutasteride in oils because of its low water solubility.7 Since this type of formulation generally shows issues such as limited stability, a short shelf life, and a reduced release rate of a drug, the development of various dutasteride formulations is needed.8 To facilitate the development of various dutasteride formulations, it is important to have rapid and sensitive methods to analyze dutasteride as a part of their evaluation. Currently, the combination of liquid chromatography (LC) and a multiple reaction monitoring (MRM) assay (LC-MRM), a type of fast, sensitive, and specific liquid chromatography and tandem mass spectrometry (LC-MS/MS), is mostly employed for that purpose.9-12 While LC-MRM is considered as a common choice of monitoring dutasteride in a sample, there have been several options for its extraction from various plasma samples. Gomes
Thus, three pre-reported methods to extract dutasteride were compared and the LLE method with methyl
Dutasteride was obtained from MSN Laboratories Limited (India). Finasteride, an internal standard (IS) and HPLC grade formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC grade acetonitrile and SPE cartridges (Strata C18-E, 50 mg, 1 mL) were purchased from Burdick & Jackson (Muskegon, MI, USA) and Phenomenex (Torrance, CA, USA), respectively. All other reagents were of analytical grade except those for HPLC.
>
Conditions for liquid chromatography and tandem mass spectrometry
LC separation was performed by a LC-20 Prominence system (Shimadzu, Tokyo, Japan). The temperature of the autosampler was kept at 4℃ and components in 30 μL of each sample were separated on a Hydrosphere C18 column (5 μm, 250 × 2.0 mm, YMC, Japan) at 45℃. An isocratic mobile phase condition (0.1% aqueous formic acid solution: acetonitrile, 30%: 70%, v/v) was used at a flow rate of 0.250 mL/min and the analysis time per sample was 15 minutes. The components eluted from the column were delivered into an API 2000 triple quadrupole mass spectrometer (AB/SCIEX, Poster City, CA, USA) through a Turbospray ion source (AB/SCIEX) for multiple reaction monitoring (MRM) assays of dutasteride (528.9/460.9/49, the
>
Preparation of stock solutions, standards, and quality control samples
Each stock solution (2 mg/mL) was prepared by dissolving 2 mg of dutasteride or IS in 1 mL of methanol. Working standard solutions with certain concentrations were prepared by appropriate dilution of the stock solutions. In the case of the IS working standard solution, its concentration was 200 ng/mL. Calibration standard plasma samples and quality control (QC) samples were prepared by spiking a working standard solution in blank rat plasma (final concentrations of dutasteride: 5, 10, 20, 40, 80, 160, and 400 ng/mL). All solutions were stored at -20℃ until analysis.
To simplify components in calibration standard plasma samples, QC samples, and pharmacokinetic plasma samples, a certain extraction method should have been applied to a sample prior to its analysis. Thus, for the determination of the most suitable method to extract dutasteride and IS from a rat plasma sample, three different dutasteride extraction methods (SPE,10 LLE1 with methyl
As a result of extraction tests, LLE1 was selected as the extraction method for calibration standard plasma samples, QC samples, and pharmacokinetic plasma samples. Briefly, 100 μL of plasma was thawed and mixed with 20 μL of the IS working standard solution, 100 μL of an aqueous sodium hydroxide solution (1 mol/L), and 600 μL of a LLE reagent (methyl
The method composed of the LLE1 extraction method and the LC-MRM method were validated in terms of specificity, linearity, sensitivity, accuracy, precision, and recovery. Explanation on methods for the validation can be found in Method validation in Results and discussion
>
In vivo pharmacokinetic study in rats
The comparative
Collected pharmacokinetic plasma samples were treated and were analyzed by using the LLE1 method and the LC-MRM method, respectively. Pharmacokinetic data analysis was performed using a BA Calc 2007 pharmacokinetic analysis computer program (Korea Food & Drug Administration, Korea). Area under the curve (AUC) was calculated using the linear trapezoidal rule by the program. Maximum plasma concentration (
>
Comparisons of extraction methods
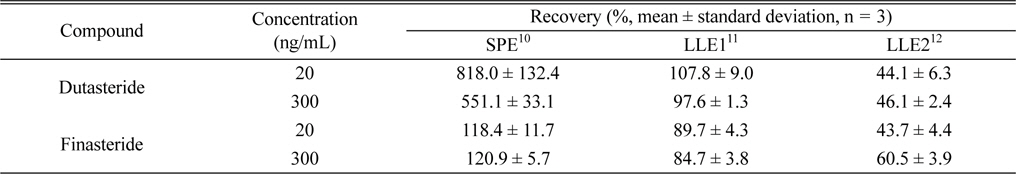
For the evaluation of individual dutasteride extraction method performances, the recovery of each method was determined by comparing dutasteride or IS peak area of an “extracted” extraction test plasma to that of its counterpart QC working standard solution. SPE method showed 118.4% and 120.9% recovery for IS, but much higher recovery (818.0% and 551.1%) than 100% for dutaseride (Table 1). These results are significantly different from previous ones (dutasteride recovery: 99.74 - 109.85%; IS recovery: 98.96 - 105.05%) and its reason might be related with the use of different SPE cartridges (Strata C18-E instead of Strata-X DVB HL) and the application to different species (rat instead of human).10 However, since any endogenous nor exogenous interfering compound was not detected from double blank rat plasma extraction using the SPE method (data not shown), more investigation is required to find out reasons of different recovery values between the literature and the present study. In the case of LLE2 method which employs methyl
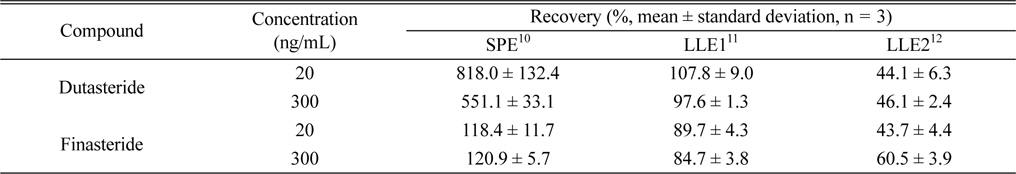
Comparisons of the extraction of dutasteride and finasteride from rat plasma using three different methods
While the LLE1 method showed good performance to extract dutasteride from rat plasma, the validation of a method to monitor dutasteride in rat plasma which includes the LLE1 extraction method has not been carried out. Thus, the method composed of the LLE1 extraction method, LC, and MRM assays for dutasteride and IS was validated by the assessment of its specificity, linearity, sensitivity, precision, accuracy, and recovery.
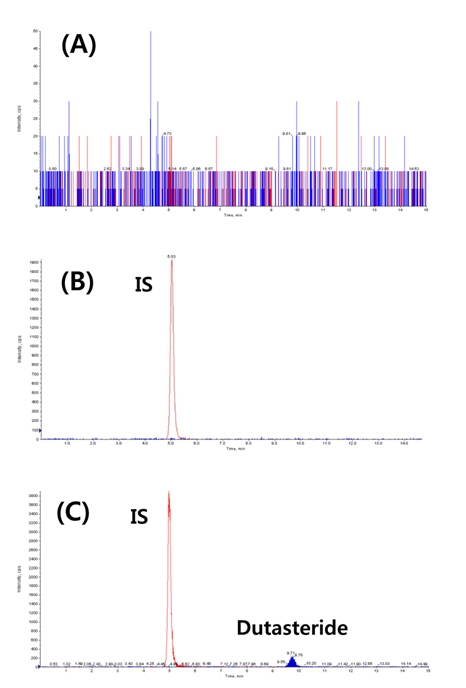
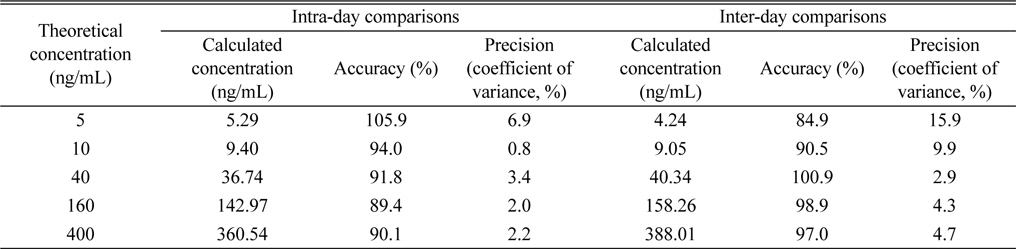
The specificity of the method was confirmed by the absence of peak representing dutasteride or IS from blank rat plasma samples (n = 6). While dutasteride and the IS were confirmed at 9.71 minutes and 5.04 minutes, respectively, from the positive control analysis, any endogenous nor exogenous interfering compound peak couldn’t be observed from blank plasma analyses (Figure 2). The good linearity (r2 = 0.9993) of the method was also observed at a dutasteride concentration range between 5 - 400 ng/mL, and the limit of detection (LOD, S/N ≥ 3) and the limit of quantitation (LOQ, S/N ≥ 10) were calculated as 4.03 ng/mL and 12.10 ng/mL, respectively. Its precision and accuracy were studied by using QC samples (n = 5) at 5 different concentrations (5, 10, 40, 160, and 400 ng/mL). Accuracy and precision were expressed as the percentage of a calculated concentration based on a calibration equation to its theoretical concentration and the coefficient of variance value among replicate results, respectively. Intra-day comparisons were done within a day and inter-day comparisons were carried out over five consecutive days. As shown in Table 2, the intra-day accuracy, the inter-day accuracy, the intra-day precision, and the inter-day precision were determined as 89.4 - 105.9%, 84.9 - 100.9%, 0.8 - 6.9%, and 2.9 - 15.9%, respectively. As mentioned above (Comparisons of extraction methods in Results and discussion), the recovery range of the method was determined as 84.7 - 107.8% (Table 1).
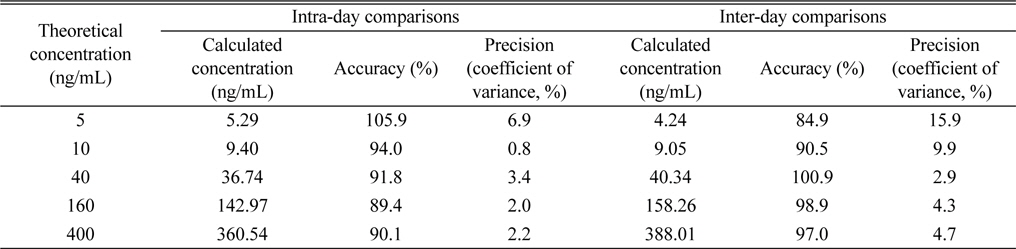
Intra-day and inter-day comparisons of results from LC-MRM analyses of dutasteride in rat plasma
>
In vivo pharmacokinetic study
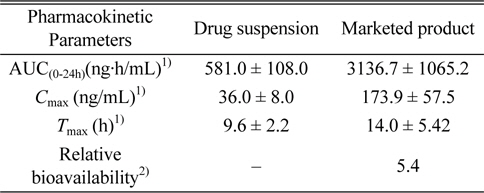
The validated method was applied to pharmacokinetic studies in rats to determine the drug concentration in rats after oral administration. Figure 3 presents the plasma concentration-time profile of the 5-α-reductase inhibitor in rats after oral administration of drug suspension and marketed product at a dose equivalent to 2 mg/kg. And the pharmacokinetic parameters, including

The pharmacokinetic parameters of dutasteride from the drug suspension and marketed product (Avodart®) after a single oral administration in rats at a dose of 2 mg/kg
Three pre-reported methods for dutasteride extraction were compared and the LLE method using methyl