Abalone immortal cell lines can be used to study the physiological properties and disease mechanisms of abalone at the cellular and molecular level. As a first step for the final goal to establish abalone immortal cell lines, we examined various initial culture conditions for primary cell populations derived from Haliotis discus hannai radula tissue. The survival rate after cell isolation procedures using the enzymatic method was as low as 9.95 ± 2.37%. Based on three different experimental conditions for H. discus hannai radula-derived cell culture, we found that the salinity of the media and the presence of growth-promoting factors were important to support radula-derived primary cell populations during the initial culture. The growth factor-containing media adjusted to 35 psu salinity could induce 100% (8 out of 8 trials) initial cell attachment, and the rate of cell attachment reached 50–70%. The data obtained from this study will provide useful information for developing immortal cell lines from abalone species.
Abalone is a valuable food source worldwide, and farming of abalone is common in numerous countries (Cook and Gordon, 2010). To improve the productivity and the quality of individuals, physiological properties of the abalone should be characterized at the cellular and molecular level. Moreover, the mechanism of diseases that can result in death
Abalones (
>
Radula tissue collection and cell isolation
For tissue collection, healthy adult abalones were sterilized using 70% ethanol (SK Chemicals, Sungnam, Korea) for 2 min. Radula tissues were removed from the bodies using sterilized surgical equipment and washed three times in Dulbecco’s phosphate-buffered saline (DPBS; Gibco, Grand Island, NY, USA). For cell isolation, radula tissues were placed in 35-mm petri dishes (SPL life Sciences, Pocheon, Korea) filled with digestive medium consisting of DPBS supplemented with 500 U/mL collagenase type I (Worthington Biochemical Corporation, Lakewood Township, NJ, USA) and 0.05% trypsin–EDTA (Gibco), minced using a surgical blade, and incubated for 30 min at room temperature. After enzyme inactivation by adding 3 mL of 10% (v/v) fetal bovine serum (FBS; Cellgro, Manassas, VA, USA)-containing Leibovitz’s L-15 medium (L15; Cellgro) or the high glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco), all tissue derivatives were filtered through a 40-μm cell strainer (SPL Life Sciences) and retrieved by centrifugation at 400 ×
The isolated cells were seeded in a well of 0.1% gelatin (Sigma-Aldrich, St. Louis, MO, USA)-coated 48-well plates (Thermo Scientific, Vernon Hills, IL, USA) or 96-well microplates (Thermo Scientific) filled with culture media. The basic culture media were L15 (designated as L15-basic) or 386DMEM buffered with HEPES (designated as DMEM-basic) supplemented with 15% (v/v) FBS, 15% (v/v)
The cell survival rate after the cell isolation procedure employing an enzymatic method was 9.95 ± 2.37% from four replicates [48/504 (live/total) × 104 cells = 9.52% in replicate 1, 48/612 (live/total) × 104 cells = 7.84% in replicate 2, 48/528 (live/total) × 104 cells = 9.09% in replicate 3, and 40/300 (live/total) × 104 cells = 13.33% in replicate 4]. Several factors may contribute to the low cell survival rate. For tissue digestion, enzymatic treatment is commonly used (Lian et al., 2003; Hinsch and Zupanc, 2006; McIntosh et al., 2006), but types of enzymes and their concentration may also affect cell survival. In addition, low salinity of the digestive medium may provoke cellular shock since
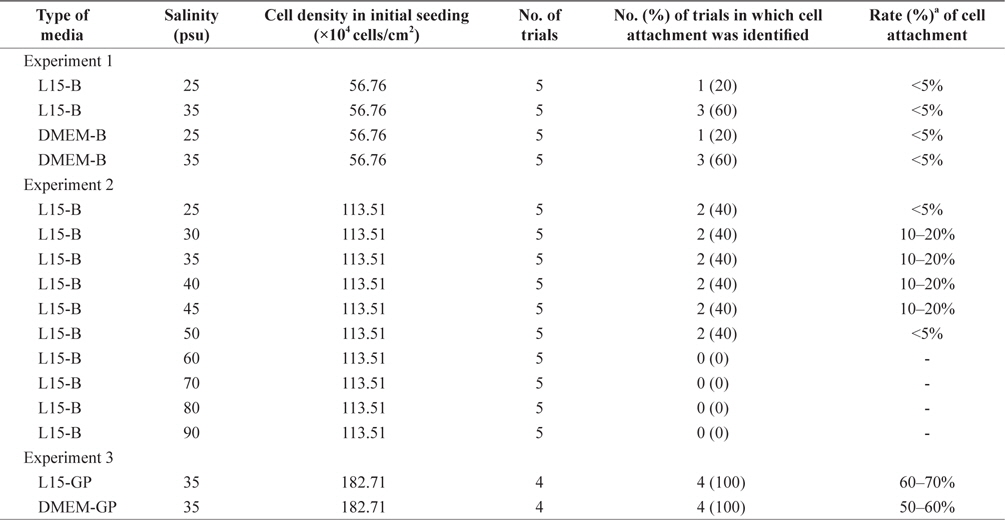
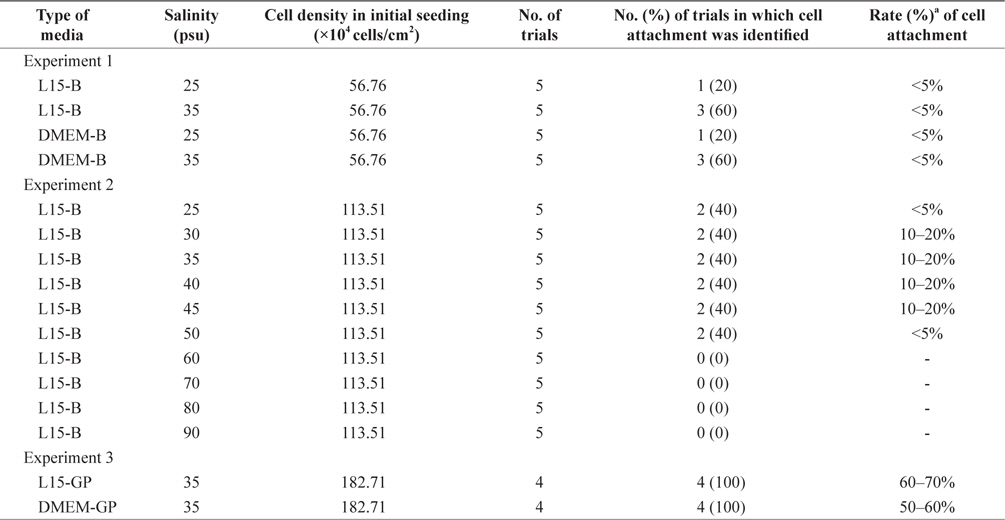
Next, we cultured the isolated cells under three different experimental conditions to derive the primary cell population (Table 1). In experiment 1, L15-basic or DMEM-basic medium adjusted to either 25 or 35 psu were used for cell culture. No significant differences were detected among the treatment groups based on the number of trials in which the initial cell attachment was identified (p = 0.3909). Regardless of experimental treatments, 40% of trials (8/20) induced initial cell attachment, but the attachment rate was less than 5% in all cases. In this study, initial cell attachment and the rates were used as the final outcomes to evaluate primary culture success because cell attachment allows us to evaluate cell survival and growth based on simple microscopic observations. Additionally, because almost all cell types derived from animals have been cultured using an attachment-based culture method, the measures used in this study are deemed appropriate. Nevertheless, considering that several cell types grow well in a suspension manner (Bellamy et al., 2001; Sen et al., 2002; Gammell et al., 2007), another strategy to target nonattached cells in the initial culture is required. The basal salinity level of media was 25 psu, in which most animal and plant cells can grow normally. However, based on different physiological properties between marine invertebrates and terrestrial organisms, different conditions may be required for
[Table 1.] Culture outcome of primary cells derived from Haliotis discus hannai radula
Culture outcome of primary cells derived from Haliotis discus hannai radula
In experiment 2, two factors including initial cell density and salinity were differentially applied to the initial culture of
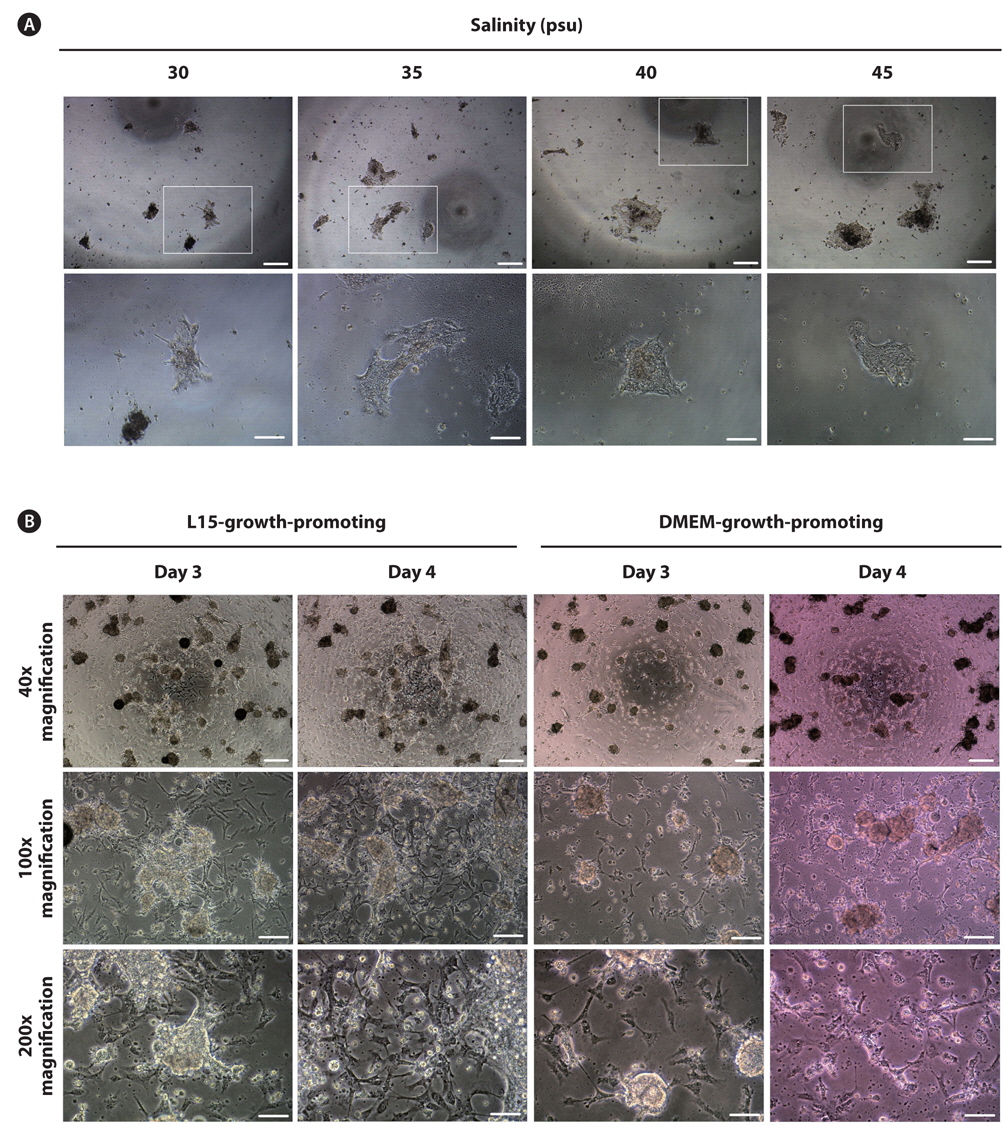
As a final experiment, we used media containing growthpromoting factors under a fixed salinity of 35 psu. Two types of media including L15- and DMEM-growth-promoting media were used, and the initial cell density was further increased 1.6-fold compared with experiment 2. Significant improvement was derived from experiment 3. All eight trials (4/4 = 100% in L15-growth-promoting and 4/4 = 100% in DMEM-growth-promoting) successfully induced initial cell attachment with rates of 60–70% and 50–60% in L15- and DMEM-growth-promoting media, respectively. As shown in Fig. 1B, many cells were attached to the bottom of the plate as aggregates or a single cell in both media types at day 3 of culture. Based on visual observation, growth of these cell populations was detected at day 4 of culture regardless of the media type (Fig. 1B), which were continuously retained without significant change up to day 9 of culture. For one cell population cultured in L15-growth-promoting medium, the first subculture was successfully progressed at day 7 of culture, but the cells could not survive beyond 2 days after subculture. Under this experimental condition, the culture medium was additionally supplemented with various components to promote cell growth based on the composition of fish stem cell culture media (Yi et al., 2010; Lee et al., 2013). Specifically, bFGF, EGF, and embryo extracts are known to promote cell proliferation in culture (Collodi and Barnes, 1990; Raballo et al., 2000; Kimura et al., 2013). As expected, the initial culture outcomes of
In conclusion, initial culture for primary cell populations derived from