Aquaculture is the fastest growing food producing sector in the world today, and demands for feed ingredients, especially fish meal and oil, have increased dramatically in recent years (Hardy et al. 2001, National Research Council 2011, Food and Agriculture Organization of the United Nations 2014). Macroalgae have successfully been incorporated into fish and shrimp feeds at levels up to approximately 100 g kg-1 feed without compromising growth or survival (Mustafa et al. 1995, Valente et al. 2006, Khan et al. 2008, Ergün et al. 2009). Occasionally, increases in feed consumption and growth have been reported. Red sea bream Pagrus major growth improved with a 50 g kg-1 addition of the green alga Ulva pertusa, the brown alga Ascophyllum nodosum, or the red alga Pyropia yezoensis (see AlgaeBase for authorities; Guiry and Guiry 2014) to the feed, although the largest weight gain was observed with the red alga (Mustafa et al. 1995). Ergün et al. (2009) reported an increase in the growth of Nile tilapia Oreochromis niloticus when Ulva rigida was added at a level of 50 g kg-1 feed while Stadtlander et al. (2013) reported an increase in growth in tilapia when P. yezoensis was added at 136 g kg-1, but not when added at 272 g kg-1.
Gracilaria and related genera comprise about 200 species of warm water to tropical seaweeds that are widely distributed throughout the world, except the polar seas (McLachlan and Bird 1984, Lüning 1990, Yang et al. 2012, Guiry and Guiry 2014). Gracilaria is a bushy, branching seaweed, irregularly or dichotomously branched, and may have rounded, compressed, or flattened axes (Lüning 1990). Blades are usually red, but can be brownish, green, or almost black depending on light and nutrient conditions (Kim and Yarish submitted). Gracilaria has a wide range of tolerance to nutrients, salinity, and temperature (Lüning 1990, Yokoya et al. 1999, Choi et al. 2006, Thomsen and McGlathery 2007). The genus is common to estuaries and bays and is often found in intertidal or shallow subtidal areas, less than 1 m deep, either attached to rocks or free floating. It is also found in embayments that may be rich in inorganic nitrogen and phosphorus (~55 μmol L-1 of nitrogen and ~19 μmol L-1 of phosphorus) (Hanisak 1990, Yarish et al. 1991, Teichberg et al. 2008, Kim et al. 2014). The native Gracilaria tikvahiae and its invasive congener Gracilaria vermiculophylla are commonly found along the northeastern United States coastline and the two are virtually indistinguishable, except by genetic markers (Saunders 2009, Kim et al. 2010, Nettleton et al. 2013). G. tikvahiae is a euryhaline species, which can tolerate a wide range of salinities, 15-60 psu, though it grows best from 15-38 psu (Bird and McLachlan 1986). It can survive temperatures of 10-30℃ but has an optimal range of 20-25℃ (Bird et al. 1979, McLachlan and Bird 1984).
At present, the annual global harvest of Gracilaria is about 1.7 million metric tons with an annual value of $540 million USD (Yang and Yarish 2011). Gracilaria has historically been a commercial source of food grade and biotechnological grade agar, and its economic value is very stable (Yarish and Pereira 2008). Beyond its traditional uses, Gracilaria may be suitable for biofuel production (Notoya 2010), an ingredient for fish feeds (Mustafa and Nakagawa 1995, Kanjana et al. 2011, Fleurence et al. 2012) and employed for nutrient bioextraction in integrated multi-trophic aquaculture (IMTA) systems (Abreu et al. 2009, 2011a, 2011b, 2011c, Yarish and Kim 2013, Kim et al. 2014).
In the present study, we cultivated native G. tikvahiae in open water farms in urbanized estuaries of Long Island Sound and the Bronx River, New York, USA to extract nutrients from these urbanized estuaries and potentially produce a valuable ingredient for aquaculture feeds. The study sites are located within the Long Island Sound watershed and New York City’s estuary, which receive anthropogenic inputs from a population of 9 million people (Latimer et al. 2014). It was previously demonstrated that G. tikvahiae readily sequesters nitrogen and carbon from these sites (Kim et al. 2014). However, it is unknown how much sequestered nitrogen is present as protein and how nutritious this protein is for aquaculture species. As such, the objective of this study was to determine the protein and amino acid composition of G. tikvahiae harvested from these nutrient bioextraction systems over a four month harvest period and evaluate the potential of this algal protein as an ingredient for fish and shrimp feeds.
A G. tikvahiae strain (G-RI-ST1) was mass cultured at the Seaweed Marine Biotechnology Laboratory at University of Connecticut, Stamford, CT, USA. This strain was originally collected in June 2010 from Potters Pond, South Kingstown, Rhode Island, USA (41°22′56″ N, 71°32′04″ W). G. tikvahiae was out-planted on long lines at two open water farm sites, 67 km apart. The first out-planting was on July 28, 2011 in Long Island Sound (LIS; 41°4′8″ N, 73°9′10″ W), and the second out-planting was on September 20, 2011 at the mouth of the Bronx River Estuary (BRE; 40°48′5″ N, 73°52′16″ W). For each site, long lines (45 m at the BRE site and 100 m at the LIS site) were installed at 0.5 m depth as described elsewhere (Kim et al. 2014). Twenty gram bundles of G. tikvahiae thalli were inserted into nylon rope line for both sites. Three bundles were harvested on Aug 16, Sep 15, and Nov 4, 2011 from the LIS site and on Oct 19, 2011 from the BRE site for tissue analysis.
Salinity during the growing season at LIS ranged from 26-30 psu, and at BRE were slightly lower, 20-25 psu. Subsurface irradiance was measured with a LiCor LI-185A PAR meter (Lincoln, NE, USA) as described elsewhere (Kim et al. 2014). At the LIS site, mean light penetration was 81% at 0.5 m and 53% at 1.0 m deep, during mid-day on cloudless days. Mean light penetration at the BRE site was similar to the LIS site at 81% at 0.5 m and 48% at 1.0 m deep. The water temperature at both sites and at the culture depth ranged 22-24℃ (July to September) and decreased to 13℃ by early November (Kim et al. 2014). Seawater nitrogen and phosphorus content was monitored at the two sites through the automated determination of total ammonia (Bertheot method), nitrate + nitrate (cadmium reduction, azo dye method), and dissolved inorganic phosphorus (acidified molybdate method) on a QuAAtro Autoanalyzer (Seal Analytical, Mequon, WI, USA) as described by Hansen and Koroleff (1999). Nitrogen concentrations at the LIS site ranged 2.7-8.4 μmol L-1 while those at the BRE site were 38-55 μmol L-1 during the 2011 summer-fall growing season. Phosphorus concentrations during the same time period ranged 0.9-4.7 μmol L-1 at the LIS site and 14-19 μmol L-1 at the BRE site.
Approximately 100 g of G. tikvahiae from different clean bundles, without fouling organisms, were sampled for nutrient analysis at each harvest (n = 3). Samples were immediately dried at 55℃ and stored in a dry environment until shipment. The dried samples were shipped to the Northwest Fisheries Science Center, Seattle, WA, USA for protein extraction and constituent analyses. Upon receipt, algal samples were dried overnight in a 105℃ oven to a constant weight and then finely ground. Protein was extracted via precipitation with 5% trichloroacetic acid (TCA) as described by Woyewoda et al. (1986). Dried algae and TCA protein extracts were finely ground and sent to the University of Missouri Agriculture Experiment Station Chemical Laboratory, Columbia, MO, USA for Kjeldahl nitrogen analyses in accordance with AOAC Official Method 984.13 (AOAC International 2000). Dried algal samples were additionally analyzed for complete amino acid profiles in accordance with AOAC Official Method 982.30 (AOAC International 2000).
Total nitrogen and crude protein (CP) are expressed on a dry matter (DM) basis of the original algal sample. In accordance with AOAC methodology, the CP content of algae was estimated by multiplying protein nitrogen by a factor of 6.25. For comparative purposes, amino acid concentrations are presented both on a DM basis and a CP basis.
All statistical analyses were performed with R version 2.15.0 statistical software (The R Foundation for Statistical Computing, Palo Alto, CA, USA). One-way analysis of variance (ANOVA) was employed to detect differences in total nitrogen and protein over time with differences between means detected by Tukey’s HSD test. Differences in nitrogen content attributable to harvest date were deemed significant when p < 0.05.
One-way ANOVA was similarly employed to detect differences in amino acid concentrations on a CP basis over time with differences between means detected by Tukey’s honestly significant difference test. After applying the Bonferroni correction for multiple comparisons (n = 19) at the p < 0.05 level, differences in amino acid concentrations attributable to harvest date were deemed significant when p < 0.0026. Unless stated otherwise, results are presented as mean ± standard deviation.
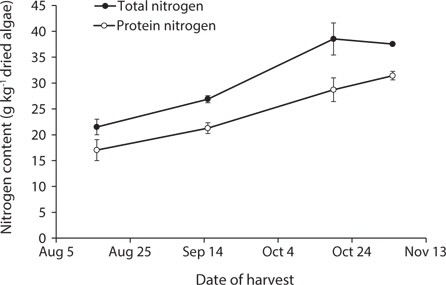
Total nitrogen and protein nitrogen increased proportionately over the harvest period (Fig. 1). On average, non-protein nitrogen (NPN) accounted for 21 ± 1% of all nitrogen present. Total nitrogen increased from 22 ± 1 g kg-1 DM in August to 39 ± 3 g kg-1 DM in October (p < 0.001), with no difference observed between October and November (p = 0.90). CP followed a similar trend and increased from 107 ± 13 g kg-1 DM in August to 196 ± 5 g kg-1 DM in November (p < 0.001) with no difference observed between October and November (p = 0.45).
Increases in amino acids mirrored that of CP (Table 1). The two most abundant amino acids at all harvests were aspartic acid and glutamic acid with mean concentrations of 102 ± 3 g kg-1 CP and 101 ± 5 g kg-1 CP, respectively. With two exceptions, amino acid concentrations expressed on a CP basis were similar over time. Tryptophan significantly increased from 6.3 ± 0.3 CP g kg-1 in August to 8.9 ± 0.9 g kg-1 CP in October (p = 0.002) with no differences observed between October and November. Conversely, cysteine significantly decreased from 30 ± 1 g kg-1 CP in August to 24 ± 2 g kg-1 CP in September (p = 0.002) and remained at this lower concentration through November.
Over the harvest period, essential amino acids accounted for 48 ± 1% of all amino acids present. Lysine and methionine were present at moderate concentrations, averaging 56 ± 2 g kg-1 CP and 18 ± 1 g kg-1 CP, respectively. Histidine was underrepresented among essential amino acids and averaged 13 ± 1 g kg-1 CP. Taurine concentrations ranged from 2.1 to 3.2 g kg-1 DM.
The nitrogen content of G. tikvahiae grown at our study sites ranged from 22-39 g kg-1 DM. This is similar to levels previously reported for Gracilaria species collected from the wild (Bird et al. 1977, Penniman et al. 1986, Lourenço et al. 2002, Abreu et al. 2011c), but less than levels reported from some shore-based IMTA systems (Valente et al. 2006, Abreu et al. 2011a). The two-fold increase in the nitrogen content of G. tikvahiae between August to November is consistent with results from a previous study with this species raised in the Great Bay Estuary, NH, USA (Penniman and Mathieson 1987) and another study with Gracilaria sp. raised in Pomquet Harbour, NS, Canada (Bird et al. 1977). The increase in protein content of G. tikvahiae from 106 to 196 g kg-1 over the harvest period, suggests that ocean farmed G. tikvahiae may be a suitable ingredient for aquaculture feeds, especially when harvested late in the season.
Amino acid concentrations, expressed on a CP basis, were relatively constant during the study. This demonstrates the conserved nature of G. tikvahiae protein over the harvest period. NPN was slightly higher than NPN reported for Pyropia sp. (15% NPN) by Dawczynski et al. (2007), but within the range (7-32% NPN) reported by Lourenço et al. (2002) for other red algal species. Compared to terrestrial grasses and legume forages routinely fed to ruminants, NPN levels in dried G. tikvahiae were higher than levels typical for fresh plants (10-15% NPN), similar to levels in hay (15-25% NPN), and lower than levels in silages (30-65% NPN) (National Research Council 2001).
The two major amino acids present in G. tikvahiae at all four harvests were aspartic and glutamic acid, which accounted for over 20% of the protein present. This is similar to values reported for other red algal species (Dawczynski et al. 2007, Gressler et al. 2010), and less that what has been reported for brown algae (Dawczynski et al. 2007, MacArtain et al. 2007). Lysine was detected in moderate amounts in G. tikvahiae and unlikely to be a limiting amino acid in feed formulations. However, histidine and methionine were present at lower concentrations and may be limiting. Low concentrations of histidine were similarly observed in four Brazilian red algae species (Gressler et al. 2010) and Porphyra and Pyropia spp. (Walker et al. 2009). Based on the National Research Council (NRC)’s “ideal protein” profiles for fish and shrimp (National Research Council 2011), histidine is likely to be the first limiting amino acid for G. tikvahiae protein in aquaculture feeds, followed by methionine (Table 2). An “ideal protein” contains a perfect balance of essential amino acids for the animal. The NRC ideal protein profiles for fish and shrimp are compiled from 15 nutritional studies spanning 10 different species and are a useful starting point for formulating aquaculture feeds. In a feeding study with juvenile Atlantic cod, Walker et al. (2009) overcame the low histidine content of Porphyra and Pyropia spp. by concomitantly adding blood meal to the experimental feeds.
The lower tryptophan concentrations observed among August plants suggests that tryptophan may additionally be limiting for feeds prepared from plants harvested in the summer. Similarly, tryptophan and histidine were the first two limiting amino acids in Gracilaria domingensis for red hake Urophycis chuss followed by methionine and lysine (Burkholder et al. 1971). By comparison, tryptophan in P. yezoensis protein was below levels required by tilapia, despite observing an increase in growth when 15% of the fish meal in their experimental feeds was replaced by P. yezoensis (Stadtlander et al. 2013.
Taurine in G. tikvahiae, 1.3-2.1% of the amino acids present, was slightly lower than values reported for Pyropia produced in Japan, but similar to levels reported for Pyropia produced in China (Dawczynski et al. 2007). Taurine levels of G. tikvahiae exceeded those previously reported for brown algae (Dawczynski et al. 2007). Taurine, an amino sulfonic acid, has important roles in osmoregulation, bile acid conjugation, membrane stabilization and calcium homeostasis in vertebrates (Huxtable 1992). The capacity of cultured fish to biosynthesize taurine depends on the species, with marine species typically unable to biosynthesize enough taurine for optimum growth. Although the underlying physiological processes are not yet understood, the addition of taurine to the diet can improve the growth of important marine aquaculture species such as olive flounder Paralichthys olivaceus (Kim et al. 2005), red sea bream (Matsunari et al. 2008), and cobia Rachycentron canadum (Lunger et al. 2007) as well as some freshwater species such as rainbow trout Oncorhynchus mykiss (Gaylord et al. 2006). While ample amounts of taurine are found in many animal proteins, including fish meal, taurine is absent from terrestrial plants. However, taurine is present in macroalgae, which makes them an attractive ingredient for alternative, plant based, aquaculture feeds (Pedersen et al. 2012).
Farming macroalgae is an economical way to remove nutrients from eutrophic coastal waters and waters around finfish farms (White et al. 2011, Kim et al. 2013, Corey et al. 2014). It was estimated that G. tikvahiae cultured in this study removed as much as 94 kg nitrogen ha-1 and 727 kg carbon ha-1 from the BRE site over a 90-day growing period (Kim et al. 2014). Algae raised in areas contaminated by anthropogenic activities; however, may additionally sequester persistent toxins present in the environment (Cheney et al. 2007, Cruz-Uribe et al. 2007, Lotufo et al. 2008). While the presence of persistent organic pollutants is typically low in algae, high levels of heavy metals in some species have raised concerns regarding the use of algae for human food and animal feeds (Almela et al. 2002, Besada et al. 2009, Yokoi and Konomi 2012). Concern heightens when algae are harvested from areas historically contaminated from industrial activity (Giusti 2001, Lotufo et al. 2008, Lorenzana et al. 2009). Because of these concerns, we are currently measuring the concentration and seasonal variability of heavy metals in G. tikvahiae harvested at the two study sites and will be presenting these results in a future article. Initial results indicate that algae harvested at the two sites will not exceed the US Food and Drug Administrations’ regulatory limits for arsenic, cadmium, mercury, and lead for animal feeds.
In conclusion, results from this preliminary study suggest that ocean farmed G. tikvahiae may be a suitable protein ingredient for aquaculture feeds, especially when harvested late in the season. With its moderate levels of lysine, methionine and taurine, G. tikvahiae has the potential of overcoming many nutrient deficiencies currently associated with terrestrial plant proteins presently used in alternative fish feeds. In addition, since macroalgae do not require fresh water for growth, their culture may be more economical and environmentally sustainable for some regions of the world than terrestrial plants.