Reactive oxygen species (ROS) including superoxide radical (·O2–) and singlet oxygen (1O2) can result from cellular exposure to various environmental stresses such as UV light and other forms of radiation, pathogens, ozone and temperature fluctuations (Scandalios, 1993; Suzuki et al., 2012). Oxyfluorfen, a diphenylether herbicide, is inhibitor of protoporphyrinogen oxidase (PPO) which catalyzes the step leading to the formation of protoporphyrin IX (Proto IX) from protoporphyrinogen IX (Protogen IX) (Jung and Back, 2005; Nandihalli et al., 1992). This inhibition results in the accumulation of Protogen IX which diffuses to the cytoplasm and is oxidized to Proto IX via peroxidase-like enzymes in membrane. Cytoplasmic Proto IX is a potent photosensitizer resulting in the formation of ROS, causing peroxidation of membrane lipids and cell death (Jacobs et al., 1991; Lee et al., 1993). Herbicides that block electron transport or act as alternative electron acceptors and increase the flow of electrons to oxygen also significantly increase the production of ROS (Powles and Cornic, 1987). The bipyridylium herbicide paraquat is a nonselective contact herbicide that acts by intercepting electrons from the photosynthetic electron transport chain at photosystem I. This reaction results in the production of bipyridyl radicals that readily react with O2 to produce ·O2– and then, through a series of reactions, produce H2O2 and the hydroxyl radical. These toxic oxygen species cause extensive lipid peroxidation leading to loss of cell membrane integrity and rapid desiccation (Babbs et al., 1989; Kunert and Dodge, 1989). The generation of ROS contributes to the toxic effects of these herbicides. This perturbation can cause excess ROS and necessitate additional defenses.
The ROS themselves are proposed to trigger necrosis, programmed cell death as well as the induction of protective mechanisms (Scandalios, 1993; Suzuki et al., 2012). The metabolism of ROS is dependent on functionally related antioxidant enzymes, particularly hemoproteins including catalase (CAT), ascorbate peroxidase (APX) and peroxidase (POD), which destroy H2O2 (Foyer and Shigeoka, 2011; Scandalios, 1993). Enzymatic antioxidant systems provide protection against the toxic effects of ROS. APX, together with SOD constitutes the major defense system against ROS in chloroplasts (Dalton et al., 1986; Foyer and Shigeoka, 2011).
The differential herbicidal effects of diphenylether herbicide and paraquat were previously studied in tobacco leaves of varying ages (Kuk et al., 2003); however, the differential mechanism for these herbicides was not clearly elucidated in major crop plants and particularly in antioxidant responses to oxidative stresses caused by different herbicidal actions. In our study, rice plants treated either with oxyfluorfen or paraquat resulted in a substantial increase in oxidative stress, as indicated by dehydration and necrotic spots on leaves. The similarities raised the question of whether oxyfluorfen exhibits the same influence not only on oxidative stress but also on antioxidant responses as in paraquat-treated plants. A concurring alteration at enzymatic antioxidant levels against excess light and possible photooxidative stress was compared between oxyfluorfen (OF)-treated plants and paraquat (PQ)-treated plants. We established the differential antioxidant mechanisms in response to oxidative stress caused by two different herbicidal actions, photodynamic damage and photosynthetic inhibition, in rice plants treated with oxyfluorfen and paraquat, respectively.
>
Plant material and herbicide application
Rice seeds (
The rice leaf tissues were treated with oxyfluorfen and paraquat as described previously (Kenyon et al., 1985; Lee et al., 1995) by cutting 4-mm leaf squares (0.1 g FW) with a razor blade and then placing them in a 6-cm diameter polystyrene Petri dish containing 5 ml of 1% sucrose and 1 mM MES (pH 6.5) with or without the herbicide dissolved in acetone. The controls contained the same amount of the solvent without the herbicide. The final concentration of acetone in all dishes was 1% (v/v). The tissues were incubated with various concentrations of oxyfluorfen or paraquat in a growth chamber at 25℃ in darkness for 12 h, and then exposed to continuous white light at 250 μmol m–2 s–1 PPFD (photosynthetic photon flux density) for 12 h. Cellular electrolyte leakage was determined periodically by the detection of electrolyte leakage into the bathing medium using a conductivity meter (Cole-Parmer Instruments) as described in Lee et al. (1995). Because of differences in the background conductivity of different treatment solutions, the results were expressed as changes in conductivity upon exposure to light.
>
Extraction of soluble protein
For antioxidant enzyme assays, frozen leaves (0.25 g) were macerated to fine powder in a mortar under liquid N2. Soluble proteins were extracted by homogenizing the powder in 2 ml of 100 mM potassium phosphate buffer, pH 7.5, containing 2 mM EDTA, 1% polyvinylpolypyrrolidone-40, and 1 mM phenylmethylsulfonyl fluoride. Insoluble material was removed by centrifugation at 15,000×
>
Assays for antioxidant enzyme activities
Ascorbate peroxidase (APX) activity was measured spectrophotometrically by monitoring the decline in A290 as ascorbate was oxidized, using the method of Chen and Asada (1989). The 3-ml reaction volume contained 100 mM potassium phosphate buffer (pH 7.5), 0.5 mM ascorbate, and 0.2 mM H2O2 at 25℃. Total Catalase (CAT) activity was determined spectrophotometrically in a 3 ml volume containing 50 mM potassium phosphate buffer, pH 7.0, containing 20 mM H2O2 by monitoring H2O2 destruction at 240 nm (Beers and Sizer, 1952). Peroxidase (POD) activity was determined specifically with guaiacol at 470 nm following the method of Egley et al. (1983). The reaction mixture contained 40 mM potassium phosphate buffer (pH 6.9), 1.5 mM guaiacol, and 6.5 mM H2O2 in a 3-ml volume.
>
Effect of oxyfluorfen and paraquat treatment on conductivity of rice plants
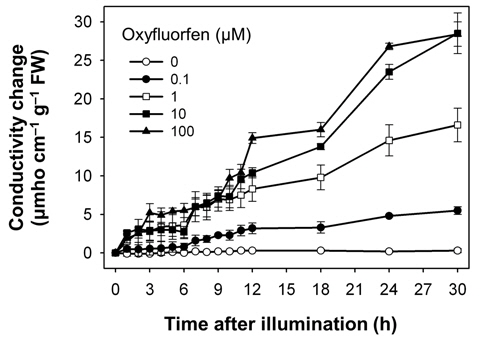
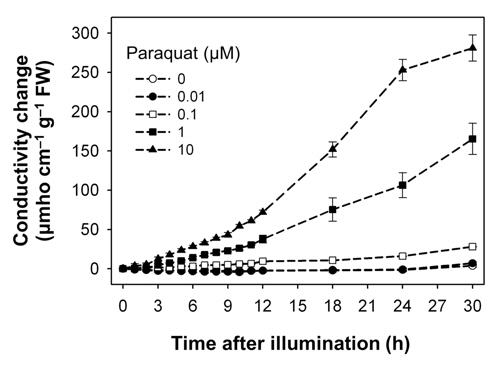
Both oxyfluorfen and paraquat are photooxidative stressinduced herbicides that block PPO enzyme and subvert electron transport, respectively (Babbs et al., 1989; Kunert and Dodge, 1989; Nandihalli et al., 1992; Powles and Cornic, 1987), and thereby resulted in desiccation and necrotic symptoms in rice plants treated with either oxyfluorfen or paraquat. We have used electroleakage as an indicator of the appearance of herbicidal response of oxyfluorfen and paraquat in the treated rice plants. The necrosis of leaf squares upon oxyfluorfen (OF) and paraquat (PQ) treatments was associated with an increase in conductivity, which indicates cellular leakage (Figs. 1 and 2). Conductivities in the OF-treated plants were increased with 0.1 μM oxyfluorfen and were further increased with higher oxyfluorfen concentrations (Fig. 1). The conductivities were drastically increased until 24 h after OF treatment and then the rates of conductivity increase were slowed down. Paraquat incubation with light for 30 h also resulted in necrosis on leaf squares of rice plants. Conductivities in the PQ-treated plants were slightly increased with 0.1 μM paraquat and were further increased with higher paraquat concentrations (Fig. 2). The conductivities were increased continuously during 30 h of illumination. The levels of conductivity were approximately 10-times higher in the PQtreated plants than in the OF-treated plants, indicating that the PQ-treated plants suffered more severe photodynamic damage than the OF-treated plants. With oxyfluorfen treatment using leaf squares, rice plants showed the increased conductivity levels, which may be due to a drastic accumulation of Proto IX in oxyfluorfen-treated plants. The necrotic phenotype of peroxidizing herbicide-treated plants displays leaf desiccation, veinal necrosis, and leaf deformation (Böger and Wakabayashi, 1999; Jung et al., 2004). The PQ-treated plants appears to increase their conductivity mainly due to the inhibited dissipation of toxic H2O2 through abnormal photosynthetic electron transport.
>
Activities of antioxidant enzymes upon foliar application of oxyfluorfen and paraquat
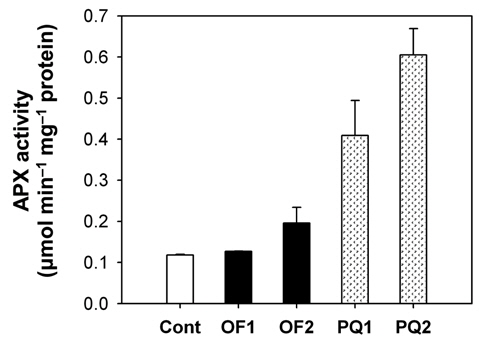
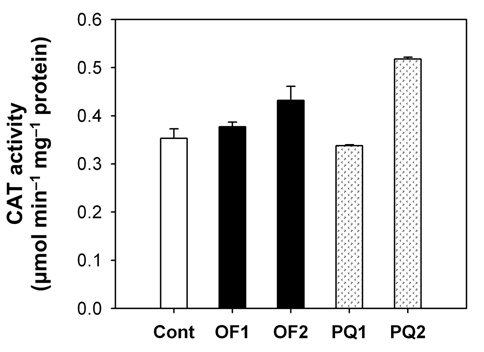
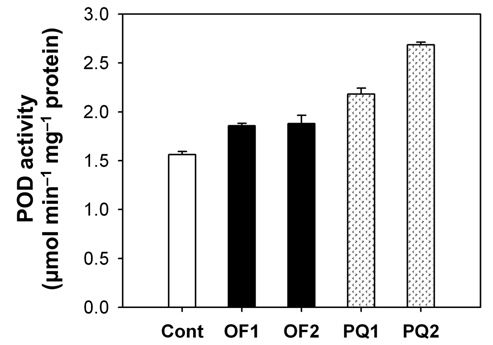
Foliar application of both 50 μM oxyfluorfen and 100 μM paraquat showed slight necrotic symptom on leaves by 1 day after those treatments and then severe necrosis and desiccation within 2 days after the treatments. However, the rice plants treated with paraquat suffered faster dehydration symptom but fewer necrotic spots, compared to the plants treated with oxyfluorfen. It has been proposed that the mechanism of herbicide resistance is a result of increased levels of enzymes that detoxify active O2 species (Jung and Back, 2005). Contribution of enzymatic antioxidant responses, which are mediated by increasing amounts of ROS upon herbicidal treatment, was compared in OF- and PQ-treated rice plants. Total activities of all antioxidant enzymes including APX, CAT and POD increased upon both oxyfluorfen and paraquat treatment compared with those of untreated control plants (Figs. 3-5). At 1 day after the administration of 50 μM oxyfluorfen, rice plants did not exhibit any noticeable change in enzyme activities of APX (Fig. 3). APX activity was noticeably increased in rice plants at 2 days after oxyfluorfen treatment. Total APX activity exhibited 3-fold increase 1 day after 100 μM of paraquat treatment and further increased 2 days after the treatment (Fig. 3). APX utilizes ascorabate as a specific electron donor to reduce H2O2 to water with the concomitant generation of monodehydroascorbate, a univalent oxidant of ascorbate (Foyer and Shigeoka, 2011). In the OF- and PQtreated plants, total activities of CAT were not noticeably changed at early stages of the treatments, but considerably increased 2 days after the herbicide treatments (Fig. 4). The maximum increases in CAT activity were observed on day 2 in the PQ-treated plants. CAT is known to take part in an efficient protective role against oxidative stress (Chaoui et al., 1997). In response to the two herbicide treatments, total POD activity increased at 1 day after the treatment and then increased further in both OF- and PQ-treated plants, in comparison to untreated control plants (Fig. 5). The paraquat treatment resulted a greater increase in POD activity than that of the oxyfluorfen treatment. The enzymatic removal of H2O2 by POD and CAT seems to be the dominant pathway during oxyfluorfen- and paraquat-induced enzymatic scavenging system in rice. Overall, paraquat treatment resulted in marked increase in antioxidant enzyme activities in comparison to those of oxyfluorfen treatment, implying that the PQ-treated plants developed stronger defense responses against the oxidative stress.
ROS produced in plants are normally detoxified by enzymatic and non-enzymatic antioxidants present in all cellular compartments, especially at sites where oxygen radicals are usually photogenerated (Foyer et al., 1994; Jung, 2011). Increased levels of the enzymes that detoxify active O2 species have been implicated in protective mechanism. The photooxidative stress caused by either oxyfluorfen or paraquat appears to trigger the dissipative process, enzymatic antioxidants including APX, CAT, and POD. Various antioxidant enzymes appear to play an essential part of defense mechanisms against oxyfluorfenand paraquat-induced oxidative stress. The combined action of an H2O2-scavenging enzyme, CAT and POD, is critical in mitigating the effects of oxidative stress (Foyer and Shigeoka, 2011; Scandalios, 1993; Suzuki et al., 2012). On the basis of differences in antioxidant responses of rice plants to the two different herbicide applications, we suggest that the mechanism of resistance to oxyfluorfen in OFtreated plants must differ from that in PQ-treated plants.
>
Differential antioxidant mechanisms between oxyfluorefn and paraquat
The way to achieve the herbicidal symptoms appears to be different between the two herbicides, oxyfluorfen and paraquat. Oxyfluorfen mainly produces 1O2, whereas paraquat produces H2O2 in plants (Babbs et al., 1989; Jacobs et al., 1991; Kunert and Dodge, 1989; Lee et al., 1993). The mechanism for plant resistance to oxidative stress may involve increased antioxidant activities, thereby resulting in alleviated damage in plants; however, this was not clearly elucidated. When plants are exposed to high-intensity light, high or low temperatures, or ozone as well as herbicides, more ROS are generated than the scavenging mechanisms can detoxify (Alscher et al., 1997). In our study, an improved adaptive capacity of the antioxidative pathway for the detoxification of oxidative stress was generated during the oxyfluorfen and paraquat actions. Resistance to oxyfluorfen and paraquat in the rice plants is a result of the increased activities of APX, CAT, and POD in the herbicide-treated plants. However, this was not sufficient to overcome the oxidative stress, thereby resulting in severe herbicidal response in both OF- and PQ-treated plants, particularly in the PQ-treated plants. The similarity of the results obtained with paraquat and oxyfluorfen suggests that the antioxidant enzymes might provide resistance to the oxidative stress caused by both herbicides in rice plants. However, the results of these experiments demonstrate that paraquat caused not only more severe oxidative stress, as indicated by a greater change in conductivity, but also greater increases in antioxidant responses, compared with those of oxyfluorfen. This may indicate that the faster dehydration in PQ-treated plants led to greater alteration in plant defense system than the severe necrotic spots in OF-treated plants. We investigated differential oxidative stress and antioxidant mechanisms induced by oxyfluorfen and paraquat; how plants respond to handle oxyfluorfen and paraquat in which antioxidant enzymes are involved in these responses, and how these processes help protect crops against herbicides and consider the importance of these processes to successful weed management.