Polysiphonia sensu lato is a large taxon with more than 250 species widely distributed throughout the world (Guiry and Guiry 2014). According to the definition of Falkenberg (1901), species of Polysiphonia is characterized by the following features: 1) polysiphonous ultimate branches, 2) exogenous branching by diagonal division of subapical cells before cut-off of pericentral cells, 3) essentially similar and indeterminate branches, and 4) one tetrasporangium in each segment. Since Polysiphonia has a wide range of diagnostic morphological characteristics, the genus Neosiphonia was separated from Polysiphonia (Kim and Lee 1999). On the other hand, recent phylogenetic studies have shown that Polysiphonia sensu lato includes a heterogeneous group of genera: Boergeseniella Kylin, Bryocladia F. Schmitz in Engler et Prantl, Enelittosiphonia Segi, Lampisiphonia H. -G. Choi, Díaz Tapia et Bárbara, Leptosiphonia Kylin, Neosiphonia M. S. Kim et I. K. Lee, Strebocladia F. Schmitz, and Vertebrata S. F. Gray (Kim and Lee 1999, Choi et al. 2001, Mamoozadeh and Freshwater 2012, Bárbara et al. 2013). Most species of Polysiphonia sensu lato have a delicate habit composed of terete main axes which bears polysiphonous and segmented branches (Mamoozadeh and Freshwater 2011). Subsequent molecular studies divided Polysiphonia sensu lato into three strongly supported clades: the “Polysiphonia” group, the “Neosiphonia” group, and the “multipericentral” group (Choi et al. 2001, Bárbara et al. 2013).
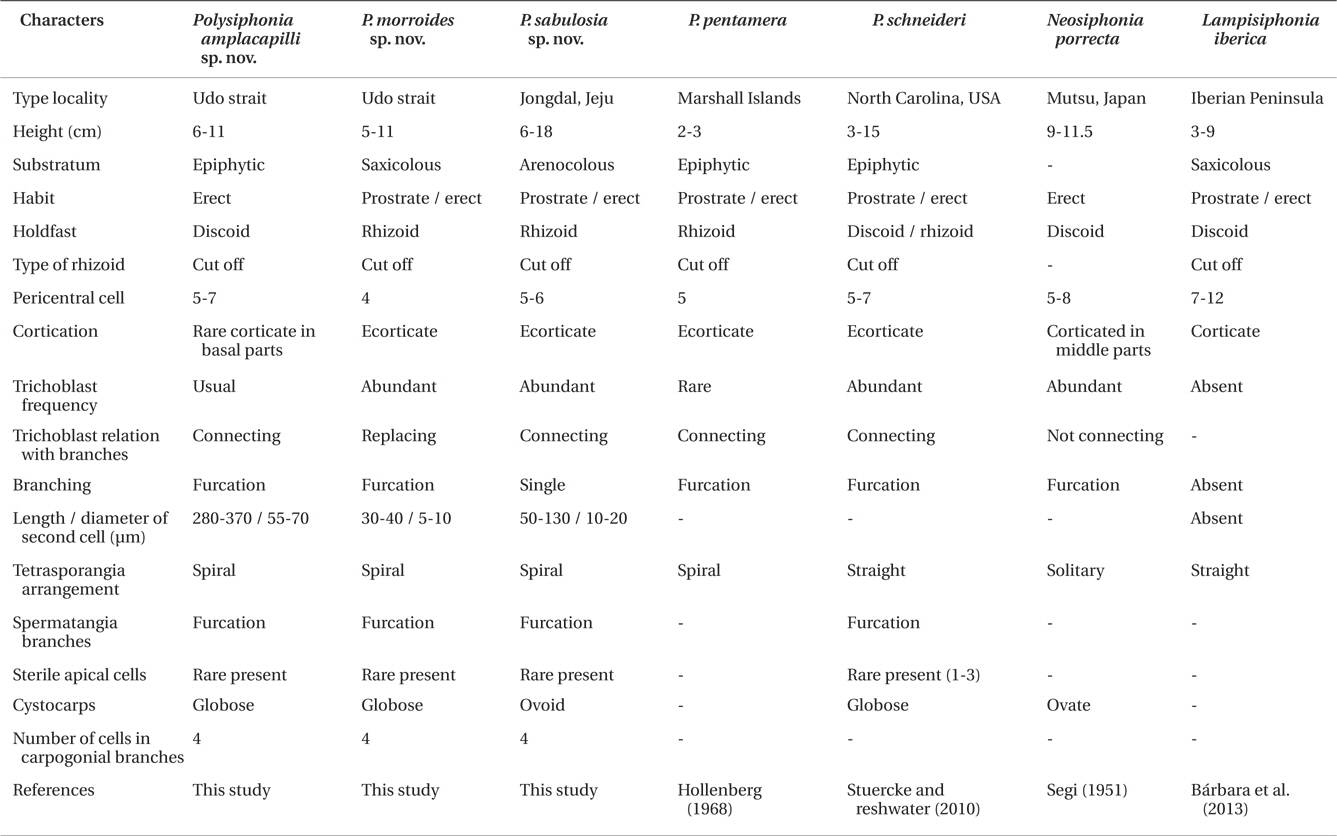
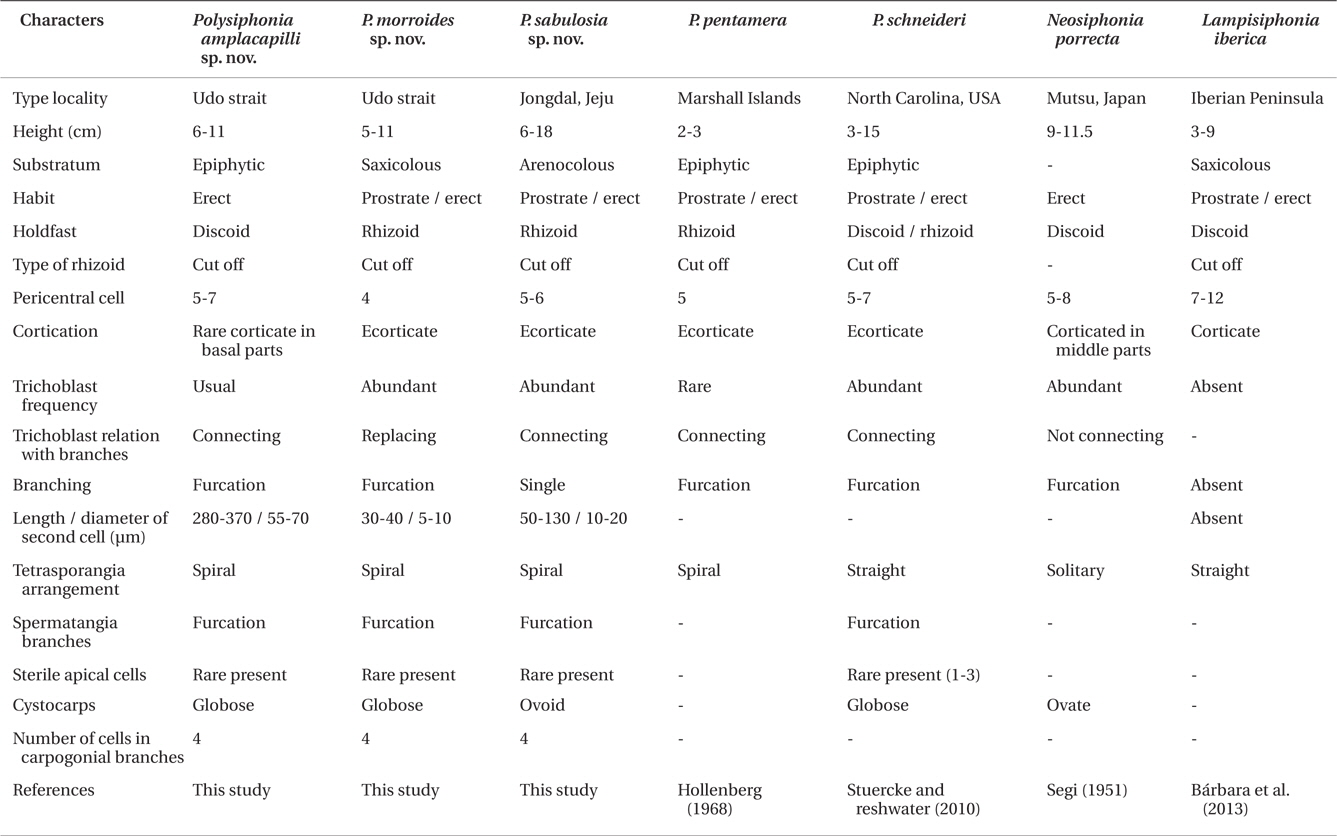
Species of Polysiphonia sensu lato has been differentiated by morphological features, such as the number of pericentral cells, type of rhizoids, presence or absence and degree of cortication, arrangement of trichoblasts, origin of branches and their relationship with trichoblast, tetrasporangia arrangement, spermatangial branches, and the number of cells in carpogonial branches (Hollenberg 1968, Kim and Lee 1999, Stuercke and Freshwater 2008). The diagnostic characteristics of the genus Neosiphonia include the production of lateral-branch initials in successive segments, rhizoids cutting off from pericentral cells, procarps with three-celled carpogonial branches, spermatangial branches arising on a branch of trichoblasts, and the spiral arrangement of tetrasporangia (Kim and Lee 1999). The new genus, Lampisiphonia, was established in Polysiphonia sensu lato through a combination of features that include the absence of trichoblasts and the presence of compact basal cortication (Bárbara et al. 2013). However, it is often difficult to establish the species delineation in Polysiphonia sensu lato based solely on morphological characteristics, as it is one of the most diverse groups of red algae. Molecular taxonomic studies have demonstrated the usefulness of DNA sequences in the species separation of Polysiphonia sensu lato. In particular, ribulose-1,5-biphosphate carboxylase and oxygenase large subunit (rbcL) has been used to identify species, as well as to analyze the phylogeny of Polysiphonia sensu lato (Stuercke and Freshwater 2010, Bustamante et al. 2012).
Ten species of Neosiphonia and twelve Polysiphonia have been reported from Korea, mostly collected in the intertidal zone (Yoon 1986, Kim and Lee 1999). However, the specimens of Polysiphonia sensu lato from subtidal habitats have been scarcely explored because of the difficulty of sampling in this environment, despite having a high possibility to discover new or unrecorded species. During our subtidal surveys (10-20 m deep) in Jeju Island, Korea, we collected three new species of Polysiphonia sensu lato. The purpose of this study is to describe their vegetative and reproductive structures, and to study their phylogenetic relationships with other species of Polysiphonia sensu lato using rbcL gene.
Specimens of Polysiphonia sensu lato were collected from the subtidal zone in Jeju Island and transported them to the laboratory using a container with ice. Samples were studied under the microscope (ECLIPS 80i; Nikon, Tokyo, Japan) and were made as voucher specimens. A piece of each thallus was taken off for molecular analysis. Sections of specimens were made with a freezing microtome (NK-101-II; Nippon Optical Works Co., Ltd., Tokyo, Japan) and were stained with 1% aniline blue acidified with 1% HCl, and mounted in 30-40% corn syrup. Photographs were taken using a digital camera (Cannon EOS 600D; Cannon, Tokyo, Japan) mounted to a microscope (Olympus BX43; Olympus, Tokyo, Japan). The voucher specimens were deposited at the Herbarium of Jeju National University (JNUB), and the National Institute of Biological Resource (NIBR), Korea.
Total genomic DNA was extracted from samples using DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) and following the manufacturer’s instructions. The specific primer pairs were used to amplify the sequence of rbcL gene: rbcLF145-rbcLR898, rbcLF7-rbcLR898, rbcLF762- rbcLR1442, and rbcLF645-rbcLR1442 (Kim et al. 2010, Kim and Kang 2011). A polymerase chain reaction (PCR) was carried out with initial denaturation at 96℃ for 4 min, followed by 35 cycles of amplification (denaturation at 94℃ for 1 min, annealing at 50℃ for 1 min, and extension at 72℃ for 2 min) with a final extension at 72℃ for 7 min (Swift MaxPro Thermal Cycler, ESCO, Singapore). The PCR products were purified using an AccuPrep PCR Purification Kit (Bioneer, Daejeon, Korea) and then sequenced commercially (Macrogen, Seoul, Korea). Both electropherogram outputs from each sample were edited using Chromas (ver. 1.45, Queensland, Australia).
Sixty-six rbcL sequences (11 new) (Table 1) were organized using the multiple-sequence editing program BioEdit (Hall 1999) and aligned visually. Herposiphonia parca, Pterosiphonia arenosa, and Womersleyella setacea were included as outgroups. Maximum likelihood (ML) analysis was performed using RAxML (Stamatakis 2006). We used the same program with the same settings and 1,000 replications to generate bootstrap values for the phylogenetic analysis (Kim et al. 2012).
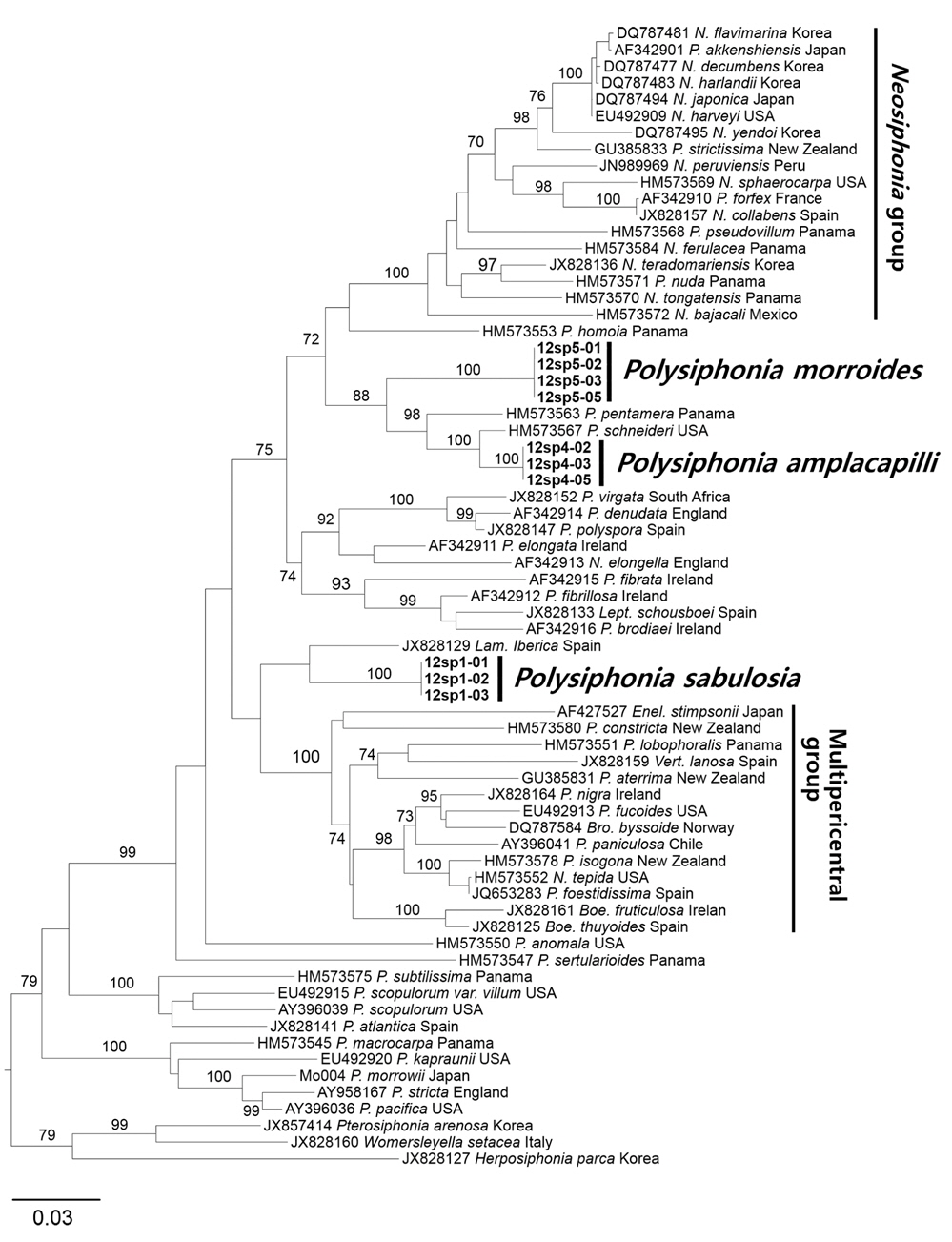
We analyzed 66 rbcL sequences of Polysiphonia sensu lato including 11 newly generated and 55 previously published, as well as three species of polysiphonous Rhodomelaceae (Herposiphonia, Pterosiphonia, Womersleyella) as outgroups. In total, 1,250 rbcL base pairs were aligned with 495 variable positions (39.6%) and 432 phylogenetically informative positions (34.6%). In the ML phylogenetic tree (Fig. 1), three new species were clearly separated from other species of Polysiphonia sensu lato. The rbcL sequences from specimens of three new species were identical from all of locations. P. amplacapilli and P. morroides were formed a same clade together with 7.8% interspecific divergence and 88% bootstrap value. But they were clearly differed from P. pentamera from Panama (HM573563) and P. schneideri from USA (HM573567) with 2.7-7.4% interspecific sequence divergence. P. sabulosia is sister to Lampisiphonia iberica from Spain (JX828129) with 5.4% divergence but no supporting value. The monophyly of each species was supported by strong bootstrap value, 100%. Three new species were clearly separated from the clade of the Neosiphonia group containing of the generitype, N. flavimarina M. S. Kim et I. K. Lee from the type locality in Korea with 72% bootstrap value. The clade of P. sabulosia is sister to the clade of the multipericentral group, but no supporting value.
Bárbara et al. (2013) reported that the monophyly of Polysiphonia sensu lato is not supported by a tree generated with Bayesian inference of rbcL data, because two genera (Odonthalia sp. and Rhodomela sp.) were resolved within a clade of the Polysiphonia sensu lato group. In our results, except for the above two genera, Polysiphonia sensu lato was also moderately supported by the bootstrap value (Fig. 1). In addition, the Neosiphonia group and the multipericentral group were strongly (100%) supported within Polysiphonia sensu lato.
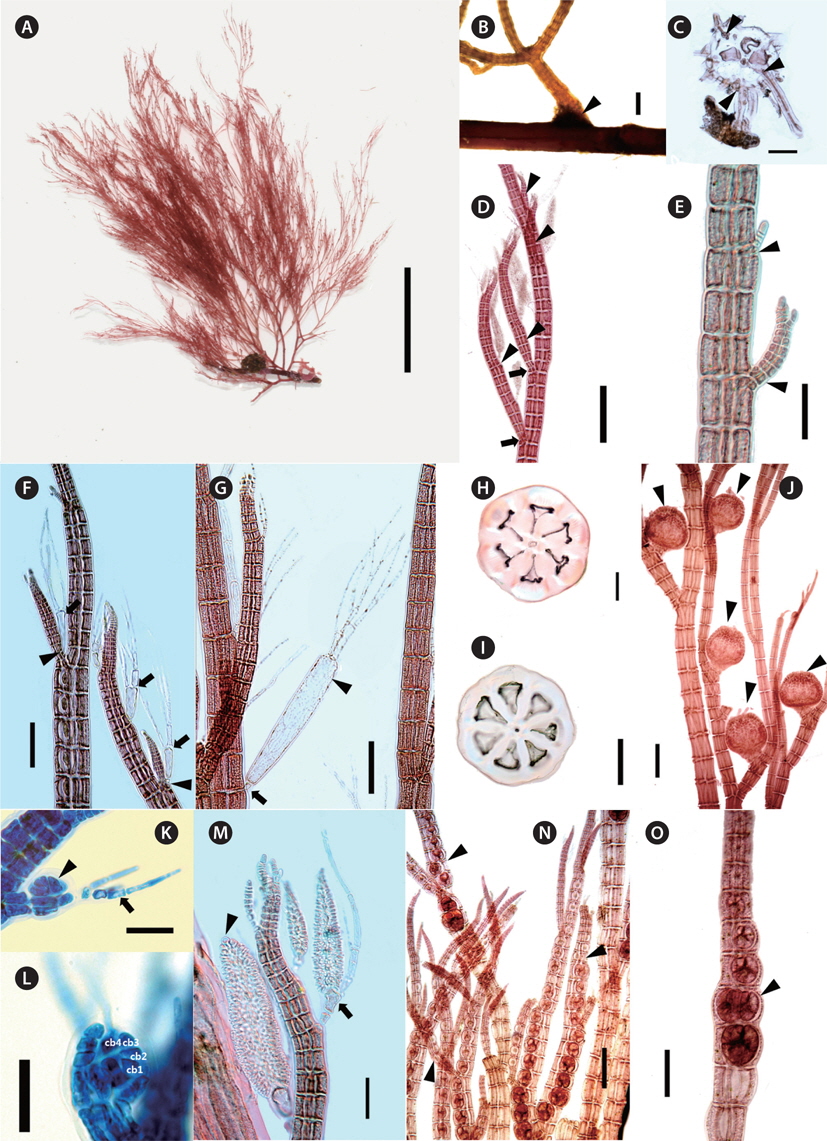
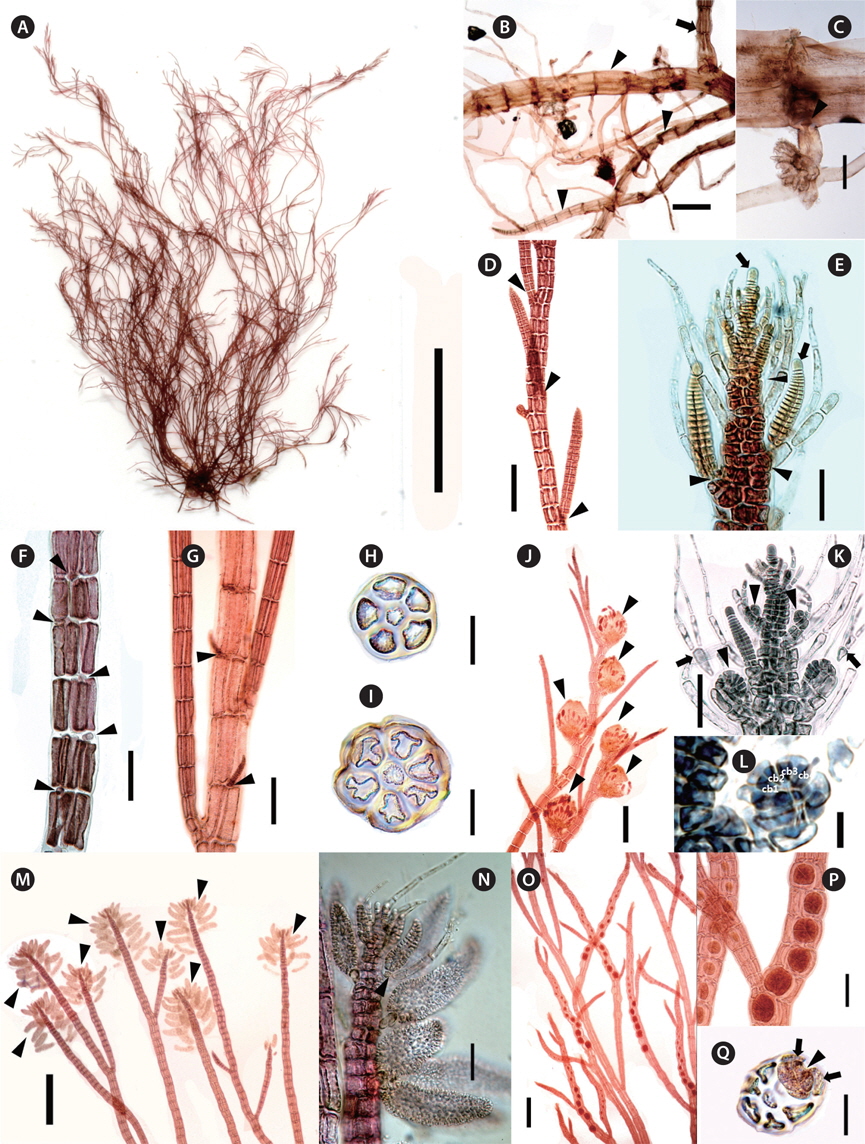
[Fig. 2.] Polysiphonia amplacapilli B. Kim et M. S. Kim sp. nov. (A) Holotype specimen (JNUB-UD120705-1, female gametophyte, July 5, 2012, Udo Strait) deposited in the Herbarium of Jeju National University (JNUB), Jeju, Korea. (B) Discoid holdfast (arrowhead). (C) Cross section of discoid holdfast with rhizoids cut-off from pericentral cells by cross walls (arrowheads). (D) Spiral lateral branches (arrowheads) constricted at their basal parts (arrows). (E) Adventitious branches (arrowheads). (F) Lateral branches arising in the axils (arrowheads) of trichoblasts (arrows). (G) Large size (arrowhead) and one small basal cell (arrow) of trichoblast. (H & I) Cross section of erect axes with 6 or 7 pericentral cells. (J) Cystocarps (arrowheads) on upper part of erect axes. (K) Developing procarp (arrowhead) and fertile trichoblast (arrow). (L) Procarp with four-celled carpogonial branch. (M) Spermatangial branches formed on one branch of first dichotomy of a trichoblast (arrow), with a sterile apical cell (arrowhead). (N) Spiral (arrowheads) arrangement of tetrasporangia. (O) Spiral arrangement of tetrasporangia (arrowhead: two tetrasporangia per segment). Scale bars represent: A, 3 cm; B, 1 mm; C, D, I, J & N, 200 μm; E-H & O, 100 μm; K & L, 25 μm; M, 50 μm.
Description. Thalli epiphytic, solitary, brownish red in color, attached by a disc consisting of a dense cluster of rhizoids which are cut off from the pericentral cells; erect axes with 5-7 pericentral cells, corticate only in the basal part; pseudodichotomously branched, lateral branches formed in the axils of trichoblasts, constricted at their basal part, obtuse apical cell; trichoblasts present, 1-4 times dichotomously branched, extraordinarily large in the reproductive plant; procarps bearing four-celled carpogonial branches, cystocarps globose; spermatangial branches cylindrical, developed on one of the branches of the first dichotomy of a fertile trichoblast; tetrasporangia arranged in spiral series.
Holotype. JNUB-UD120705-1, female gametophyte, collected on July 5, 2012, deposited in the Herbarium of the Department of Biology, Jeju National University, Korea (JNUB).
Isotypes. JNUB (UD120705-2-UD120705-4), NIBR (NIBRAL0000138757- NIBRAL0000138759).
Type locality. Udo Strait, Jeju Island, Korea (33°29′33.98″ N, 126°55′29.10″ E).
Etymology. The specific epithet was chosen to represent the large (‘ampla’) trichoblasts (‘capilli’) in the reproductive plants.
Other specimens examined. UD120606-1-UD120606-3 (Udo Strait, June 6, 2012), UD120610-1-UD120610-3 (Udo Strait, June 10, 2012), UD120705-8-UD120705-10 (Udo Strait, July 5, 2012).
Morphology. Plants are erect, 6-11 cm high, epiphytic, solitary, brownish red in color (Fig. 2A). They attach by a disc composed of a dense cluster of rhizoids which are cut off from the pericentral cells (Fig. 2B & C). Basal parts are slightly corticated and 0.7-1.2 mm in diameter (Fig. 2B). Erect axes have 5-7 pericentral cells (Fig. 2H & I), 200-500 μm in diameter, and gradually decreasing in diameter towards the apices (Fig. 2D). Apical cells are obtuse, 8-12 μm long, and 5-11 μm in diameter (Fig. 2E & M). Length / diameter ratio of segments is 1.2-1.5 at the lowers, 1.8-2.4 at the middle ones and 0.5-0.6 at the upper parts. Branching pattern is consisted of up to six orders of pseudodichotomously divided branches, forming an angle wider than 45° in the lower parts and narrowing upward (Fig. 2A & B). Branches are terete, tapered upward, constricted basal part and curved inward (Fig. 2D & M). Trichoblasts are usually appeared, develop at irregular intervals of 5-8 segments in the upper parts of erect axes. They are 1-4 times dichotomously branched (Fig. 2G), 420-620 μm long, and become extraordinarily large in the reproductive plants (Fig. 2F & G). The second cell of large trichoblasts is 280-370 μm long and 55-70 μm in diameter (Fig. 2G). The lateral branches are produced spirally in the upper part of erect axes (Fig. 2D) and connected with trichoblasts during development at segments. Scar cells appear rarely (Fig. 2F). Adventitious branches often occur in the middle and lower parts (Fig. 2E).
Procarps develop on a fertile trichoblast and they have four-celled carpogonial branches (Fig. 2K & L). Cystocarps are globose and 450-520 μm in diameter (Fig. 2J). Carpospores are clavate, 100-140 μm long and 40-50 μm in diameter. Spermatangial branches develop on the fertile trichoblasts in upper parts of erect axes. They are cylindrical, colorless, and have two basal cells that are 270-500 μm long and 50-70 μm in diameter (Fig. 2M). Sterile apical cells usually are absent, but sometimes a single sterile apical cell was observed. Fertile trichoblasts are dichotomously branched twice (Fig. 2K & M). Tetrasporangia are formed on the lateral branches on the upper parts and are arranged in spiral series, usually one per segment, sometimes two (Fig. 2N-O). They are tetrahedrally divided, 90-100 μm in diameter, and are surrounded by two presporangial cover cells. The specimens were collected at 15 m depth as epiphytic on Gracilariopsis sp. and Sargassum sp.
Remarks. P. amplacapilli is characterized by having large trichoblasts, 5-7 pericentral cells, rhizoids cut-off from pericentral cells and four-celled carpogonial branches. In particular, the large trichoblasts of this species were distinguished from other species of Polysiphonia sensu lato (Table 2). Large trichoblasts are also reported on Polysiphonia banyulensis from France, but the number of pericentral cells in P. banyulensis is four (Coppejans 1975), while P. amplacapilli has 5-7 pericentral cells. In the molecular analysis, P. amplacapilli is a sister with the Neosiphonia group (Fig. 1), but the four-celled carpogonial branches in the procarps of P. amplacapilli differed from Neosiphonia having three-celled carpogonial branches (Kim and Lee 1999). Polysiphonia schneideri Stuerck et Freshwater from USA (HM573567) and P. pentamera Hollenberg from Panama (HM573563) are closely related to P. amplacapilli as a sister group. P. schneideri was originally described from North Carolina, USA and Stuercke and Freshwater (2010) described on the species which is lacking cortication and having the secondary prostrate axes. In contrast, specimens of P. amplacapilli are characterized by the basal cortication and single holdfast. The type locality of P. pentamera is the Marshal Islands and it is different in having prostrate or decumbent branches, and not constricted at the base of branches (Hollenberg 1968). In addition, they are distinguished from P. amplacapilli since P. schneideri and P. pentamera are no large trichoblasts. Polysiphonia homoia Setchell et Gardner is the basal node related to the Neosiphonia group (Fig. 1), and the sequence divergence of P. amplacapilli was 9.9% with P. homoia from Panama (HM573553). P. homoia was originally described from Guadalupe Island, Mexico having three or five pericentral cells and 2-3 sterile apical cells in spermatangial branches (Setchell and Gardner 1930, Hollenberg 1968), whereas P. amplacapilli is having 5-7 pericentral cells and absent or one sterile apical cell.
Two species, Neosiphonia teradomariensis (Noda) M. S. Kim et I. K. Lee and Neosiphonia porrecta (Segi) Y. P. Lee having more than four pericentral cells were reported in Korea which are growing on other algae (Kim and Lee 1999, Lee 2008). N. teradomarensis is characterized by the absence of trichoblasts, 6-8 pericentral cells, and 1.5-3 cm high (Noda 1971), but P. amplacapilli is 6-11 cm high with large trichoblasts. The sequence divergence between P. amplacapilli and N. teradomarensis from Korea is 11.1%. N. porrecta has abundant trichoblasts near the apices, 5-8 pericentral cells, cortication at the middle to the basal part of erect axes, and branches independent from trichoblasts (Segi 1951). Therefore, N. porrecta differed from P. amplacapilli having large trichoblasts in the reproductive plants, branches in the axils of trichoblast and the cortication restricted to the basal part.
[Fig. 3.] Polysiphonia morroides B. Kim et M. S. Kim sp. nov. (A) Holotype specimen (JNUB-UD120610-11, tetrasporophyte, June 10, 2012, Udo strait) deposited in the Herbarium of Jeju National University (JNUB), Jeju, Korea. (B) Prostrate axes (arrowheads) with erect branches (arrows). (C) Unicellular rhizoids cut off from pericentral cells of prostrate axes by cross walls (arrowheads). (D) Cross section of prostrate axes with four pericentral cells separated rhizoids by cross walls (arrowhead). (E) Erect axes attaching by rhizoids (arrowheads) arising from other erect axes in the middle parts. (F) Spiral lateral branches (arrowheads) constricted at the basal part of branches. (G) Apical parts (arrows: apical cells) and furcated trichoblast (arrowhead). (H) Cross section of erect axes with four pericentral cells. (I & J) Procarps (arrowheads) with four-celled carpogonial branch. (K) Cystocarps (arrowheads) in upper part of erect axes. (L) Spermatangial branches formed on one branch of first dichotomy of a trichoblast (arrow), with a sterile apical cell (arrowheads). (M) Spiral arrangement of tetrasporangia. (N & O) Cross section of tetrasporangial branch (arrowhead: tetrasporangia, arrows: cover cells). Scale bars represent: A, 2 cm; B, 2 mm; C, G & L, 50 μm; D, K & M, 100 μm; E & F, 200 μm; H-J, N & O, 30 μm.
Description. Thalli saxicolous, ecorticate, attached by unicellular rhizoids which are cut off from pericentral cells, discoidal hapteron; bright brownish red in color; prostrate and erect axes terete, with four pericentral cells; branches alternate, borne at intervals of 5-7 segments; trichoblasts abundant, 3-4 times dichotomously divided, scar cell rare; procarps bearing four-celled carpogonial branches, cystocarps globose; spermatangial branches cylindrical, developed on one-time branching fertile trichoblasts, one sterile apical cell; tetrasporangia arranged in spiral series.
Holotype. JNUB-UD120610-11, Tetrasporophyte, collected on June 10, 2012, deposited in the Herbarium of Department of Biology, Jeju National University, Korea (JNUB).
Isotypes. JNUB (UD120610-12, UD120610-16-UD 1206- 10-31), NIBR (NIBRAL0000138760-NIBRAL0000138 762).
Type locality. Udo Strait, Jeju Island, Korea (33°30′02.56″ N, 126°55′38.14″ E).
Etymology. The specific epithet was chosen to represent the appearance of this plant, which is quite like Polysiphonia morrowii Harvey in the deep subtidal zone.
Other specimens examined. UD120606-11-UD120606- 13 (Udo Strait, June 6, 2012), UD120610-20-UD120610-31 (Udo Strait, June 10, 2012), UD120705-11-UD120705-13 (Udo Strait, July 5, 2012), CJD130604-1-CJD130604-3 (Chujado, June 4, 2013).
Morphology. Plants are 5-11 cm, softly tuft, and bright brownish red (Fig. 3A). Erect branches arise irregularly from the prostrate axes. Plants attach on the gravel or crustose coralline algae by unicellular rhizoids from prostrate axes (Fig. 3B). Rhizoids are cut off from the lower pericentral cell with 15-50 μm in diameter, and have a discoid haptera at the terminal part (Fig. 3C). Prostrate axes are terete, ecorticate, and 85-120 μm in diameter (Fig. 3D). Erect axes are terete, ecorticate, 100-170 μm in diameter and having four pericentral cells (Fig. 3H). Apical cells are obtuse, 8-10 μm long, and 10-11 μm in diameter (Fig. 3G). Length of segments in the erect axes gradually decreases towards the apices (Fig. 3F). Length / diameter ratio of segments is 3.1-3.3 at the lowers, 4.2-5.5 at the middle ones, and 1.5-1.6 at the upper parts. Branches are terete, indeterminate, and spirally alternate branches on each of 5-7 segments tapering upwards (Fig. 3F). Trichoblasts are abundant, colorless, 3-4 times dichotomously branched, and 150-270 μm long (Fig. 3G). The second cell of trichoblasts is 30-40 μm long, and 5-10 μm in diameter. Scar cells occur rarely on the branches. Lateral branches are replaced trichoblasts in development. Erect axes are attached by rhizoid arising from other erect axes in the middle parts (Fig. 3E). No adventitious branches.
Procarps form on a fertile trichoblast and consist of four-celled carpogonial branches (Fig. 3I & J). Cystocarps are globose, and 300-330 μm in diameter (Fig. 3K). Carpospores are clavate, 110-120 μm long, and 45-60 μm in diameter (Fig. 3K). Spermatangial branches are cylindrical, colorless, 180-220 μm long, and 40-50 μm in diameter (Fig. 3L). They develop on the fertile trichoblasts in the upper parts and have one sterile apical cell. Fertile trichoblasts are dichotomously branched once. Tetrasporangia are arranged in spiral series and composed of one per fertile segment of lateral branches (Fig. 3M). They are divided tetrahedrally, 70-95 μm in diameter, and surround by two presporangial cover cells (Fig. 3N & O). The specimens were mostly collected at 20-25 m depth and grown on crustose coralline algae such as gravel.
Remarks. P. morroides inhabits in the fast-flowing water of the subtidal zone attached to the rholith. This species looks like Polysiphonia morrowii Harvey, when we first saw it in 20-25 m depth in Udo Strait. However, P. morroides clearly differed from P. morrowii; the former species is characterized by having rhizoids cutting off from the pericentral cells, spiral arrangement of lateral branches and tetrasporangia, abundant trichoblasts, spermatangial branches developing from one basal branch of trichoblasts, and obtuse apical cells (Kim and Lee 1999, Kim et al. 2004). P. morroides is allied with P. pentamera, P. schneideri, and P. amplacapilli with 9.7, 9.1, and 9% interspecific sequence divergence, respectively.
In the morphological observation, Polysiphonia sertularioides (Grateloup) Agardh from Australia resembles with P. morroides: the former species is characterized by 2-8 cm height, dense and soft tuft thallus, rhizoids cut-off from pericentral cells, ecorticate, four pericentral cells, slightly spiral, and inhabiting in the subtidal zone (Womersley 2003). P. sertularioides is absent sterile apical cells of spermatangial branches and inhabiting on other algae at 2-9 m depth, while P. morroides is having one sterile apical cell of spermatangial branches and is collected in the fast-flowing water in 20-25 m depth. In addition, P. morroides has the lateral branches replacing trichoblast, while P. sertularioides from Caribbean Panama has the branches forming in the axil of trichoblasts (Mamoozadeh and Freshwater 2012). P. senticulosa Harvey, P. subtilissima Montagne, and P. tokidae Segi are reported in Korea or Japan resembled the external morphology with P. morroides (Segi 1951, Lee and Kang 2001). However P. sabusenticulosa and P. sutilissima are characterized by typical features of the genus Polysiphonia such as open connecting rhizoids, straight arrangement of tetrasporangia, and spermatangial branches replacing the whole trichoblasts (Womersley 2003, Mamoozadeh and Freshwater 2012). P. morroides is 5-11 cm high, 100-170 μm in diameter of erect axes, and not having adventitious branches. These characteristics of P. morroides are clearly distinguished from P. tokidae in having 1.6-4.2 cm high, 480-570 μm in diameter of erect axes, and rather numerous adventitious branches (Segi 1951).
[Fig. 4.] Polysiphonia sabulosia B. Kim et M. S. Kim, sp. nov. (A) Holotype specimen (JNUB-JD120106-1, tetrasporophyte, January 6, 2012, Jongdal) deposited in the Herbarium of Jeju National University (JNUB), Jeju, Korea. (B) Prostrate axes (arrowheads) with erect branches (arrow). (C) Unicellular rhizoid cut off from pericentral cells of prostrate axes by cross walls (arrowhead). (D) Spiral lateral branches (arrowheads). (E) Lateral branches arising in the axils of single filamentous trichoblasts (arrowheads) and apical cells (arrows). (F) Spiral arrangement of scar cells (arrowheads) on every segments. (G) Adventitious branches (arrowheads). (H & I) Cross section of erect axes with 5 or 6 pericentral cells. (J) Cystocarps (arrowheads) in upper part of erect axes. (K & L) Procarps (arrowheads) with furcated fertile trichoblasts (arrows) with four-celled carpogonial branch. (M) Spermatangial branches (arrowheads) in the apical parts. (N) One or two spermatangial branches on furcated fertile trichoblasts (arrowhead). (O & P) Spiral arrangement of tetrasporangia. (Q) Cross section of tetrasporangial branch (arrowhead: tetrasporangia, arrows: cover cells). Scale bars represent: A, 2 cm; B, D, J, M & O, 200 μm; C, F, G & P, 100 μm; E, H, I, K, N & Q, 50 μm; L, 10 μm.
Description. Thalli arenicolous, ecorticate, attached by unicellular rhizoids cut-off from pericentral cells, discoidal hapteron; bright brownish red in color; prostrate axes terete, with five pericentral cells; erect axes with 5-6 pericentral cells; branches spirally alternate, constricted at the basal part, lateral branches formed in the axil of trichoblasts; trichoblasts abundant, single filamentous; procarps bearing four-celled carpogonial branches, cystocarps ovoid; spermatangial branches in the apical part, cylindrical, developed on the branches of the first dichotomy of fertile trichoblasts, sterile apical cells one or absent; tetrasporangia arranged in spiral series.
Holotype. JNUB-JD120106-1, tetrasporophyte, collected on January 6, 2012, deposited in the Herbarium of Department of Biology, Jeju National University, Korea (JNUB).
Isotypes. JNUB (JD120106-2, JD120204-1-2, JD130203-1-2), NIBR (NIBRAL0000138763-NIBRAL0000138765).
Type locality. Jongdal, Jeju Island, Korea (33°29′51.37″ N, 126°54′58.08″ E)
Etymology. The specific epithet was chosen to represent the natural habitat of the discovery sites, where plants were growing in a sandy location.
Other specimens examined. JD120106-4 (Jongdal, January 6, 2012), JD120204-1-JD120204-5 (Jongdal, February 4, 2012), JD130203-1-JD130203-8 (Jongdal, February 3, 2013).
Morphology. Plants are 6-18 cm high, bright brownish red in color with soft tufts (Fig. 4A). Erect branches arise irregularly from the prostrate axes (Fig. 4B). The prostrate axes and lower parts of the plants are covered with sand. Plants attach on sand by unicellular rhizoids from prostrate axes. Rhizoids are cut off from the pericentral cells, 35-60 μm in diameter and have discoid haptera at the terminal parts (Fig. 4C). Prostrate axes are terete, ecorticate, 240-340 μm in diameter, and consist of five pericentral cells with 360-540 μm long, and 55-100 μm broad. Erect axes are terete, ecorticate, 165-240 μm in diameter, 5-6 pericentral cells, and spirally alternate branching pattern (Fig. 4D, H & I). Apical cells are obtuse, 10-13 μm long, and 9-11 μm in diameter (Fig. 4E). Length of segments in erect axes become longer from lower toward middle part and then are shorter upwards. Length / diameter ratio of segments is 2-2.1 at the lowers, 3.2-3.8 at the middle ones, and 0.7-0.9 at the upper parts. Branches are terete, indeterminate, and tapering upwards (Fig. 4D). The basal parts of branches is constricted (Fig. 4D & M). Trichoblasts are abundant, usually borne on the every segment of the upper part, 140-210 μm long, 10-20 μm diameter, and single filamentous with several cells connected in a row (Fig. 4E). The second cell of trichoblasts is 50-130 μm long and 10-20 μm in diameter. Fertile trichoblasts are 1-3 times dichotomous branching (Fig. 4K). Trichoblasts leave scar cells on the every segment (Fig. 4F). Lateral branch initials are produced alternately on the successive segments below the apex and lateral branches are connected with trichoblasts. Adventitious branches occurred rarely in the middle parts (Fig. 4G).
Procarps develop on a fertile trichoblast and they have four-celled carpogonial branches (Fig. 4K & L). Cystocarps are ovoid, 380-420 μm long, and 280-360 μm in diameter (Fig. 4J). Carpospores are clavate, 90-110 μm long, and 35-45 μm in diameter. Several spermatangial branches appear together in the apical parts (Fig. 4M). One or two spermatangial branches develop on a primary branch of the fertile trichoblasts (Fig. 4N). They are cylindrical, colorless, 170-210 μm long, and 50-70 μm in diameter. Sterile apical cells are usually absent, but sometimes a single sterile apical cell is observed. Fertile trichoblasts are dichotomously branched in 1-3 times. Tetrasporangia are arranged in spiral series and appear one per fertile segment of lateral branches in the upper part (Fig. 4O). Tetrasporangia are tetrahedrally divided, 70-100 μm in diameter, and surrounded by two presporangial cover cells (Fig. 4P & Q). The plants were collected in the sandy location at 5-8 m depth.
Remarks. Polysiphonia sabulosia is characterized by 5-6 pericentral cells, branches connected with single strand trichoblasts, and constricted at the basal part of branches (Table 2). In the molecular analysis, P. sabulosia is allied with the genus Lampisiphonia as a sister species, which is established from the Atlantic coast of the Iberian Peninsula (Bárbara et al. 2013). The sequence divergence of P. sabulosia differed by 6.9% from L. iberica with no bootstrap value. P. schneideri is similar to P. sabusenticulosa in having six pericentral cells, rhizoids cut-off from pericentral cells, and lateral branches developing in the axil of trichoblasts, but the former species differs in having furcations of the trichoblasts, and tetrasporangia in straight series (Stuercke and Freshwater 2010). Polysiphonia flabellulata from Japan is different from P. sabulosia by former species having 1 cm high (Masuda 1995, Yoshida 1998). Polysiphonia tapinocarpa Suringar from Japan is characterized by 8-9 pericentral cells and inhabiting on the rock at the subtidal zone (Suringar 1870, Segi 1951), whereas P. sabulosia is having 5-6 pericentral cells. P. sabulosia is similar to the characteristics of the genus Neosiphonia, such as rhizoids cut-off from pericentral cells, spiral arrangement of tetrasporangia, and abundant trichoblasts (Kim and Lee 1999), but this species has four-celled carpogonial branches as the characteristic of the genus Polysiphonia. As a result, P. sabulosia should be in the genus Polysiphonia at the present time and we need to study on classification of the species having more than four pericentral cells in Polysiphonia sensu lato.
The subtidal zone around Jeju Island could be a hotspot of species diversity for the red algae. For five years, we are continuously surveying the subtidal habitats to discover cryptic species and as a result, we collected three new species of Polysiphonia sensu lato from 10-20 m water depth from Jeju Island, Korea. However, it was difficult to identify species in Polysiphonia sensu lato, as it to one of the most diverse group of the red algal genera. To define the taxonomic entity precisely, we analyzed the morphology and the plastid rbcL gene sequences because many taxonomic molecular analysis have conducted as evidence for a species level designation. Three species are clearly distinguished from other species of Polysiphonia sensu lato in the phylogenetic tree with 7.8-14.4% interspecific divergence. We described three new species, Polysiphonia amplacapilli sp. nov., Polysiphonia morroides sp. nov. and Polysiphonia sabulosia sp. nov.