Gracilaria is one of the world’s most cultivated seaweeds with over 0.8 million tons of annual production and nearly $160 million annual values (Food and Agriculture Organization of the United Nations 2014). Most of the biomass is used in the phycocolloid industry as the main source of food grade agar (Pereira and Yarish 2008) and as a feed for animals (Qi et al. 2010, Johnson et al. submitted). Gracilaria contributes approximately 66% of the total agar production (Pereira and Yarish 2008). Gracilaria has been cultivated mainly in three different ways, including open water, pond or tank cultures (Hanisak 1987, Abreu et al. 2011, Kim et al. 2014). In general, both open water and pond cultures require a nursery (tank culture) system to provide sufficient seedstock through vegetative propagation. One important advantage of tank cultivacultivation is the ease of controlling the culture system (Pereira et al. 2013). This ensures that production meets high quality standards and biosafety for human consumptions, as well as for other high value applications such as cosmeceutical or pharmaceutical products. A limitation for tank culture, however, is high management costs (Hanisak and Ryther 1984, Caines et al. 2014).
One way to overcome this challenge is to cultivate seaweed at high stocking densities. However, Lapointe and Ryther (1979) reported that the pH in culture tanks of Gracilaria tikvahiae when grown at a high stocking density increased to ca. 9.0, which resulted in the reduction of yields. Studies on Chondrus crispus revealed that the first inorganic element to be depleted in tank cultures is inorganic carbon, due to rapid absorption of CO2/HCO3- by C. crispus, which resulted in a rapid rise in pH to values of 9-9.5. At this pH photosynthesis becomes grossly impaired because of carbon limitation (Craigie and Shacklock 1989). It is known that inorganic carbon is limiting, dependent on the seawater pH in intensively cultured tanks of G. tikvahiae (DeBusk and Ryther 1984). Bidwell and McLachlan (1985) reported that 1 g of fresh weight of G. tikvahiae placed in 100 mL of aerated sea water resulted in rapid reduction of dissolved inorganic carbon from 2.1 to 0.19 mM in 2 h.
When cultivated in a flow through system (55 L tanks at a stocking density of 2-4 g L-1), the highest yield of G. tikvahiae was found at conditions with vigorous aeration and rapid exchange of nutrient enriched seawater (no carbon, nitrogen or phosphorus limitation, or pH increase). The biomass yield was directly proportional to the seawater exchange rate (Lapointe and Ryther 1978, Hanisak 1987, Abreu et al. 2011). However, supplying high seawater exchange rates is very energy-intensive (Hanisak and Ryther 1984). Indeed, pumping seawater to provide high seawater exchange rates is the single most expensive operating cost for any land-based cultivation system (Huguenin 1976, Abreu et al. 2011).
In a land based integrated multi-trophic aquaculture (IMTA) system growing C. crispus and Palmaria palmata in Atlantic halibut effluent, pH was maintained at a low level (7.2-7.9) even at a high stocking density 40 g L-1 (Kim et al. 2013a, Corey et al. 2014). This result suggests that a land based IMTA system could be a good way to resolve the pH problem. Fish effluent also provide sufficient nutrients for the growth of seaweed (Neori et al. 2000, Martínez-Aragón et al. 2002, Matos et al. 2006, Kim et al. 2007, Corey et al. 2013). The use of fish effluent for seaweed cultivation requires that particulate matter be filtered before entering the seaweed tanks. This requires installation and maintenance costs for the filter system. In addition, seaweed grown in fish effluent may not be marketable directly for human consumption due to heavy loads of sediments, feces and fouling epiphytes. For example, in Hawaii, Gracilaria cultured in shrimp farm effluent drainage ditches or in tanks with finfish effluent from milkfish (Chanos chanos) or mullet (Mugil cephalus) were transferred to clean water on the south reef of Molokai (an island in Hawaii) for a 1-2 week depuration period before harvest (Nagler et al. 2003, Ryder et al. 2004).
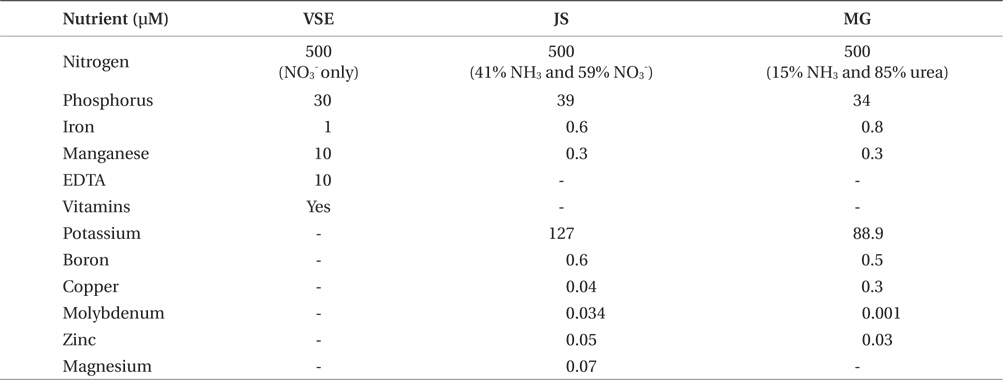
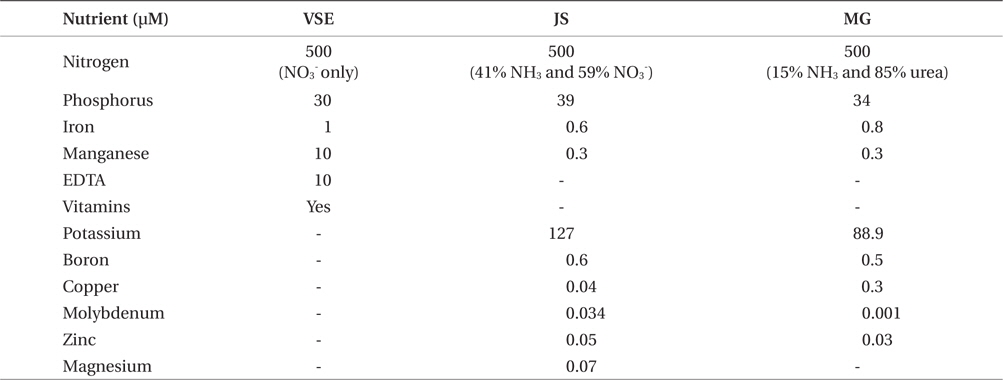
An alternative way to overcome costs associated with tank cultivation systems is to use cost efficient resources, such as injecting CO2 and using commercial fertilizers. Addition of CO2 is a common method to regulate pH, thereby preventing carbon limitation. Previous studies showed CO2 enhancement led to increased growth in Gracilaria (Gao et al. 1993), Chondrus (Craigie and Shacklock 1989), and Porphyra (Friedlander and Levy 1995, Israel et al. 1999, Friedlander 2001, Figueroa et al. 2010). The commonly used nutrient media for indoor cultivation of red seaweeds is von Stosch enrichment medium (VSE) (Corey et al. 2012, Kim et al. 2012, 2013b, Redmond et al. 2014). VSE contains several important nutrients including nitrate, phosphate, iron, manganese, EDTA, and vitamins (vitamin B12, thiamine, and biotin). However, VSE is not applicable for commercial scale seaweed cultivation due to its high material and preparation costs. In the present study, we evaluated whether or not CO2 injection, coupled with high photosynthetically active radiation (PAR) and high stocking density, could enhance G. tikvahiae production. We also evaluated two commercially available fertilizers to determine if the management costs for Gracilaria cultivation can be reduced without a decrease in productivity.
G. tikvahiae McLachlan (GT-MA5-ST2) used in this study was in unialgal culture at the Marine Biotechnology Laboratory of the University of Connecticut at Stamford. The strain of G. tikvahiae was originally collected from Waquoit Bay, MA (70°34′41.32″ N, 70°31′36.23″ W). G. tikvahiae thalli were isolated and vegetative propagated in the laboratory. This Gracilaria strain was genetically typed per the techniques of Nettleton et al. (2013). G. tikvahiae thalli (approximately 5 cm in length) were cultured in 1 L flasks at 20°C, 30 ppt of salinity, and 12 : 12 L : D photoperiod for 3 weeks. Two PAR conditions (optimal PAR, 100 μmol m-2 s-1; Bird et al. 1979 vs. 250 μmol m-2 s-1) were used to determine if the higher PAR can reduce the self-shading effect when cultivated at a higher stocking density. The medium used in the experiments was VSE seawater (von Stosch 1963; as modified by Ott 1965). Seawater was autoclaved prior to VSE addition. The stocking densities included low (0.5 g L-1) and high (10 g L-1). CO2 (Part #: CD FG50; Airgas, Radnor, PA, USA) was added to half of the cultures to regulate pH levels in the culture medium to approximately 8.0 (± 0.2), while controls remained without addition of CO2. Each flask was aerated with the appropriate pCO2 level of air, which was mixed in 13 L Pyrex jars by combining filtered ambient air and varying levels of CO2. All thalli were acclimated at the same conditions for 7 days prior to the experiment. The experimental design is a split-split plot design with PAR as the main plot, stocking density as the sub-plot and CO2 addition as the sub-sub plot (n = 5).
The culture medium was changed every other day to avoid nutrient depletion. At 1-week intervals, all of the biomass in each culture vessel was weighed for growth and productivity analyses. The biomass in each flask, with approximately 5 cm long branches, was restored to the initial stocking density each week. Samples were taken for dry weight and tissue analysis. For the analysis of tissue nitrogen (N) and carbon (C) contents, samples were dried in an oven at 55℃ to constant weight (ca. 48 h) and later ground to a uniform powder (Model MM200 Grinder; Retsch, Haan, Germany). The powder was analyzed using a Perkin Elmer 2400 series II CHNS/O elemental analyzer (Perkin Elmer, Wellesley, MA, USA).
Due to limitations in seedstock of G. tikvahiae strain of GT-MA5-ST2, another unialgal and molecularly identical strain was used in this experiment (Nettleton et al. 2013). G. tikvahiae (G-RI-ST1; originally collected from Potters Pond, RI (41°23′45.36″ N, 71°32′12.52″ W) was cultivated in 1 L flasks at 20°C, 30 ppt of salinity, 100 μmol m-2 s-1 of PAR and 12 : 12 L : D photoperiod for 3 weeks. Since vitamins are included in VSE but not in commercially available fertilizers, five media conditions were utilized: 1) VSE, 2) Jack’s Special only (JS; N : P : K, 21 : 8 : 18; product #77440; J. R. Peters, Inc., Allentown, PA, USA), 3) JS with vitamins (JSV), 4) Miracle-Gro only (MG; N : P : K, 24 : 8 : 16; The Scotts Miracle-Gro Co., Marysville, OH, USA), and 5) MG with vitamins (MGV). The stocking density was maintained at 2 g L-1. Total nitrogen and phosphorus concentrations in each medium were adjusted to be the same as those in VSE (500 μM and 30-39 μM, respectively) (Table 1). However, the nitrogen sources were different in each medium (VSE, 100% NO3; JS, 41% NH3 and 59% NO3; MG, 15% NH3 and 85% urea). The experimental design was a randomized complete block design with nutrients as the factor. All other conditions and measurements were same as above in the ‘effects of CO2 injection.’
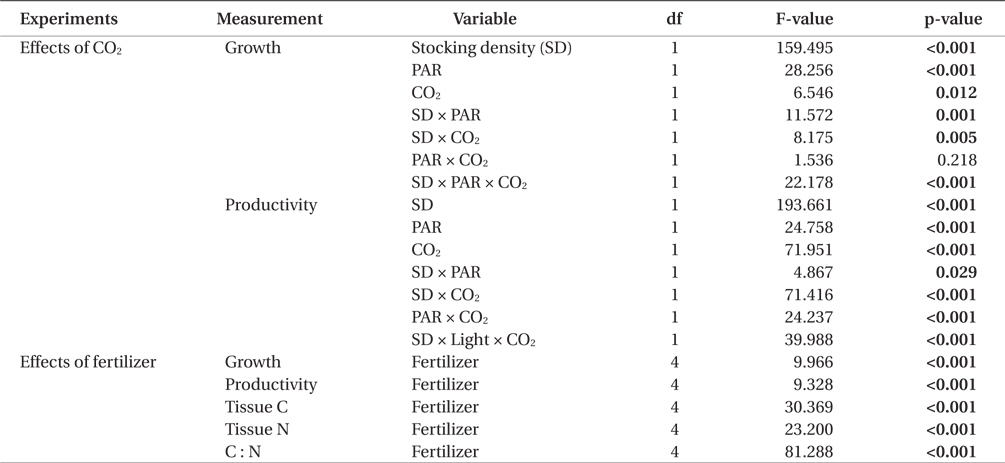
For the ‘effects of CO2 injection’ experiments, repeated measures ANOVA (α = 0.05) was used to analyze growth and productivity. For this analysis, time was the within- subject factor; PAR, CO2, and stocking density were between-subject factors. Repeated measures ANOVA (α = 0.05) was also used to analyze growth in the ‘effects of commercial fertilizers’ experiments. For the analysis of tissue N and C contents and C : N ratio, two-way or one-way ANOVA was used for the ‘effects of CO2 injection’ experiments and the ‘effects of commercial fertilizers’ experiments, respectively. Tukey’s HSD analysis (α = 0.05) was used as a post hoc test to determine pairwise comparison probabilities between treatment level means. All analyses were conducted using IBM SPSS Statistics ver. 20 (IBM, Armonk, NY, USA).
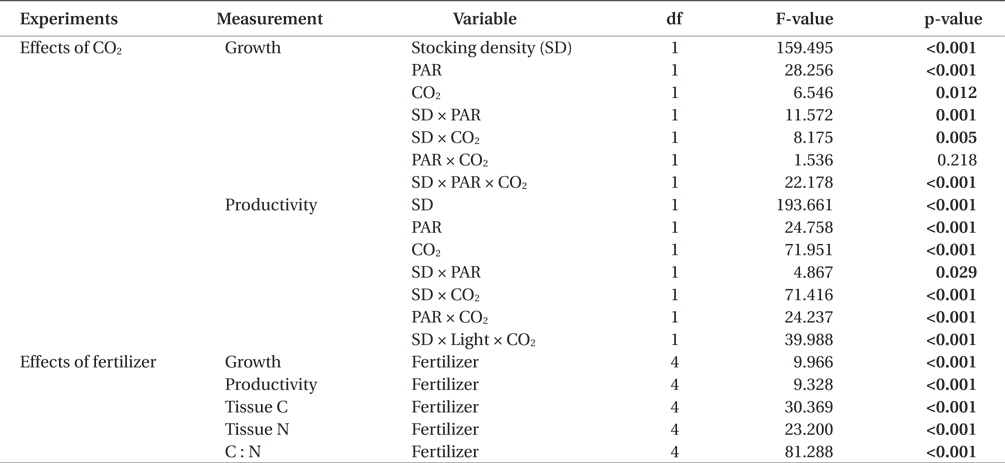
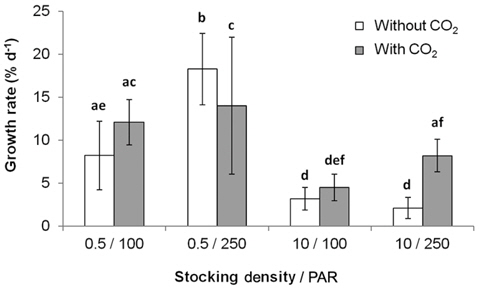
All three variables (stocking density, light, and CO2), and all combinations of these variables, except for PAR × CO2, significantly affected the growth rate and productivity of G. tikvahiae (Table 2). The highest growth rate was observed at 0.5 g of stocking density and 250 μmol m-2 s-1 of PAR without CO2 injection (18.28% d-1) (Fig. 1). As a group, low stocking density had a higher growth rate (13.16% d-1) than a high stocking density (4.51% d-1) regardless of other conditions. However, Gracilaria at the high stocking density was 4.5 times more productive (0.57 g L-1 d-1) than lower stocking density cultures (0.13 g L-1 d-1) (Fig. 2). In addition, with all other factors held constant, high stocking density cultures at high PAR receiving CO2addition (1.13 g L-1 d-1) were 2.1 times as productive as those receiving a low PAR (0.54 g L-1d-1). When considering both high stocking density and high PAR, the effect of CO2 addition yielded productivity rates 4.8 times greater than the cultures under the same conditions without CO2 injection (Fig. 2). Tissue C and N contents, and C : N ratio were not significantly different at different conditions (p > 0.05). The values were 27.7-31.2% DW for C, 2.9-3.6% DW for N, and 8.5 : 1-10.5 : 1 for C : N, respectively (data not shown).
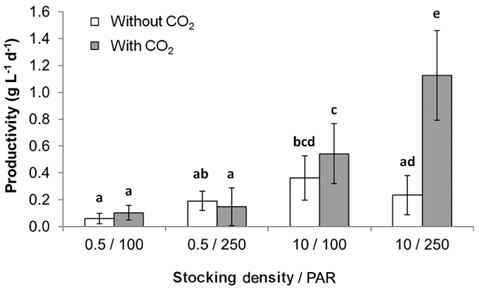
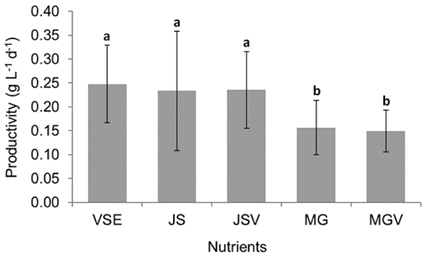
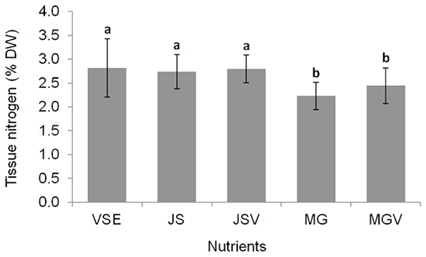
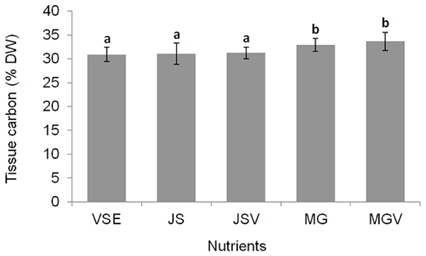
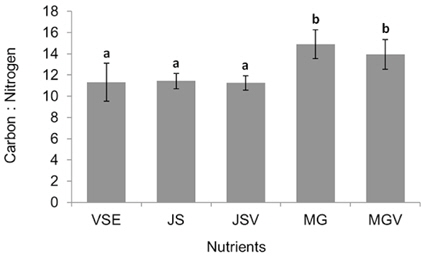
All metrics (growth, productivity, tissue C and N contents, and C : N ratio) were significantly affected by nutrient conditions (p < 0.001) (Table 2). Vitamins did not affect any of metrics (p < 0.001). Gracilaria showed similar responses to VSE, JS, or JSV in all metrics while the plants grown in MG, and MGV had significantly lower values for these metrics (Figs 3-5). The average growth rate (on average 8.4% d-1) and productivity (on average 0.24 g L-1 d-1) of Gracilaria grown in VSE, JS, and JSV were 29% and 36%, respectively, higher than those in MG and MGV (6.0 % d-1 of growth rate and 0.16 g L-1 d-1 of productivity). The relative tissue N content was also on average 16% higher in VSE, JS, and JSV (on average 2.78% DW) than that in MG and MGV (on average 2.34% DW). However, the tissue C content and C : N ratio were higher in MG and MGV (on average 33.29% DW and 14.4 : 1, respectively) than the other three conditions (on average 31.08% DW and 11.3 : 1, respectively) (Figs 6 & 7).
Our study demonstrated that the use of high stocking density and high PAR, coupled with CO2 enhancement, may be most efficacious option to optimize productivity of G. tikvahiae in land-based nursery cultivation systems. Seaweed with a high stocking density is usually self-shading, therefore reducing photosynthetic and growth rates of the seaweed (Kim et al. 2013a, Corey et al. 2014). This could result in the reduction of growth, and therefore the reduction of production. In the present study, however, although the low stocking density showed a higher growth rate of Gracilaria than at a higher stocking density, the productivity at the higher stocking density greatly exceeded that at the lower stocking density. At the high PAR, CO2 injection and high stocking density, productivity was nearly 6 times higher than that at the most rapidly growing lower stocking density condition. This combination also out-produced its control group without receiving additional CO2 by a factor of 4.8.
The primary cause for the significant productivity increase in the CO2 enhanced high stocking density, high PAR group, was probably due to increased carbon availability for photosynthesis (Lapointe and Ryther 1979). The productivity of the high stocking density high PAR without additional CO2 was only one fifth of that of the CO2 enhanced, high stocking density, high PAR group. This result suggests that carbon limitation, and therefore reduction of photosynthesis, was the cause for this difference. Such an explanation is consistent where lower growth rates were observed at pH levels above 9.0 (DeBusk and Ryther 1984). Evidence for this is that in the present study, the observed pH range of the lower stocking density, under PAR of 250 μmol m-2 s-1, was 8.3 to 8.9. The growth rates at this lower stocking density at high PAR were not significantly affected by CO2 addition, suggesting carbon limitation was not an issue for this group. At the higher stocking density cultures without CO2 injection, the pH was in the range of 9.1-9.6. High alkalinity causes trace elements such as iron to form less soluble metal hydroxides (Bidwell and McLachlan 1985), thus decreasing the bioavailability of essential nutrients needed to sustain long-term high productivity (Craigie and Shacklock 1989). In Chondrus crispus, a pH above 9.0 induced necrosis after 14 days (Simpson et al. 1978).
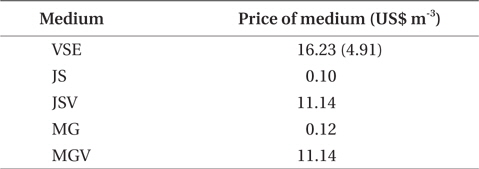
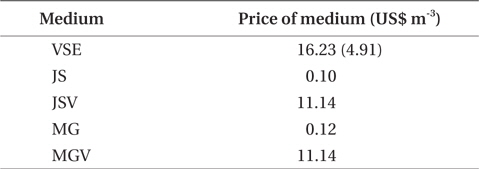
We also found that a commercially available fertilizer, Jack’s Special (N : P : K, 21 : 8 : 18; product #77440; J. R. Peters, Inc.) can replace VSE to grow Gracilaria in land based cultivation systems. The Gracilaria cultivated in the medium with JS fertilizer grew as well as the plant in VSE. The growth rates and productivities of G. tikvahiae in VSE, JS, and JSV were similar, 8.2-8.7% d-1 of growth rates and 0.23-0.25 g L-1 d-1 of productivity, respectively, while the growth rates and productivities at MG and MGV were significantly lower than those in other conditions. It was probably because of a higher portion of urea in MG. Organic N, including urea, may contribute substantially to macroalgal N demand; however, the uptake rate of urea by seaweeds including Gracilaria vermiculophylla is low (Phillips and Hurd 2004, Tyler et al. 2005). This might have caused the slower growth and lower tissue N contents in G. tikvahiae with MG and MGV than with VSE, JS, and JSV. Vitamins did not show any significant effect on the growth and productivities of this species. When the price of each medium was compared, JS proved to be the least expensive ($0.1 per m3 of medium) than VSE ($16.23) (Table 3). Although the costs for vitamins were removremoved from VSE, the cost for JS media is still 2% of the cost for VSE without vitamins.
Commercial fertilizers have been used in small scale seaweed cultivation research or on an industrial scale (Enright and Craigie 1981, Tyler et al. 2005, Alan Critchley, Acadian Seaplants Ltd. personal communication). However, most of these studies, if not all, did not provide detailed information on the commercial fertilizers used in their studies. It is probably because the studies formulated their own mixture of nutrients, and / or the formulation was proprietary. The two fertilizers tested in this study can be easily purchased off the shelf (e.g., Home Depot, Atlanta, GA, USA; Lowes, Mooresville, NC, USA; or garden supply stores, etc.) or online (e.g., http://www. jrpeters.com; http://www.miraclegro.com). The findings from this study suggest a potential use of a commercially available fertilizer (e.g., JS) in land-based Gracilaria cultivation systems.
The present study showed that CO2 injection improved the productivity of Gracilaria and that a commercial fertilizer can replace the expensive chemical based nutrients in land-based Gracilaria cultivation systems. Combining high stocking densities of Gracilaria and high irradiance, would maximize the utilization of space and energy. It is important to note that the findings from this study were based on one liter scale experiments using fluorescent light. To apply the finding from this study to large scale cultivation systems, there may be technical and / or economic challenges. LED lighting or ambient sunlight is the most cost efficient source of light for indoor or outdoor, respectively. When different sources of light were used, the optimal PAR conditions must be determined for each light source. It is also important to determine appropriate cultivation conditions (e.g., an optimal stocking density, media change frequency, aeration, etc.) to avoid any potential negative impact of waste (unused fertilizer) to local environments. An appropriate CO2 injection method must also be determined based on the cultivation system (e.g., tank or pond) (Craigie and Shacklock 1989). There is also a need to study the economic analysis for CO2 addition. One way to reduce the costs for CO2 is using the CO2 from power plants or industrial processes (Mata et al. 2010). Inorganic nutrients have been primarily used in land based Gracilaria cultivation systems. However, organic fertilizers can even enhance the economic value of products and maybe enable the Gracilaria product to receive an organic food designation (Harrison 2008).