Novel baculovirus vector systems recombined with coding genes of polyhedron promoter, vesicular stomatitis virus G (VSVG), polyA, cytomegalovirus (CMV) promoter, enhanced green fluorescent protein (EGFP), and protein transduction domain (PTD) were constructed. These recombinant baculovirus vector systems were applied into human foreskin fibroblast cells and compared the effects of gene transfer and gene expression of these recombinant baculovirus vector systems with control vector system. From this study, it showed that these novel recombinant baculovirus vector systems were superior efficacy to control vector system in view of gene transfer and gene expression.
폴리히드론 프로모터, 수포성구내염 바이러스G, 폴리A, 사이토메가바이러스 프로모터, 강화녹색형광단백질, 단백질전달부위 유전자 등을 포함한 새로운 베큘로바이러스 시스템이 제조되었다. 본 재조합벡터 시스템은 인간 섬유아세포에 적용하여 시험하였고 재조합된 유전자의 전달과 유전자 발현을 대조 벡터시스템과 비교하였다. 본 연구로부터 새롭게 제작된 본 베큘로바이러스 시스템이 유전자의 전이와 유전자 발현 면에서 대조 벡터시스템 보다 고효율을 나타내었다.
The baculoviruses are a family of large rod-shaped viruses that can be divided to two genera: nucleopolyhedroviruses (NPV) and granuloviruses (GV). While GVs contain only one nucleocapsid per envelope, NPVs contain either single (SNPV) or multiple (MNPV) nucleocapsids per envelope. The enveloped virions are further occluded in granulin matrix in GVs and polyhedrin for NPVs. Moreover, GV have only single virion per granulin occlusion body while polyhedra can contain multiple embedded virions [1].
Baculoviruses have very species-specific tropisms among the invertebrates with over 600 host species having been described. Immature (larval) forms of moth species are the most common hosts, but these viruses have also been found infecting sawflies, mosquitoes, and shrimp. Although baculoviruses are capable of entering mammalian cells in culture [2], they are not known to be capable of replication in mammalian or other vertebrate animal cells. Baculoviruses contain circular double-stranded genome ranging from 80–180 kbp.
The earliest records of baculoviruses can be found in the literature from as early as the sixteenth century in reports of “wilting disease” infecting silk-producing larva. Starting in the 1940s they were used and studied widely as biopesticides in crop fields. Since the 1990s they have been utilized for producing complex eukaryotic proteins in insect cell cultures (see Sf21, High Five cells). These recombinant proteins have been used in research and as vaccines in both human and veterinary medical treatments (for example, the most widely used vaccine for prevention of H5N1 avian influenza in chickens was produced in a baculovirus expression vector). More recently it has been found that baculoviruses can transduce mammalian cells with a suitable promoter [3]. These medical and potential medical uses have accelerated the number of publications on baculoviruses since 1995.
The baculovirus life cycle involves two distinct forms of virus. Occlusion derived virus (ODV) is present in a protein matrix (polyhedrin or granulin) and is responsible for the primary infection of the host while the budded virus (BV) is released from the infected host cells later during the secondary infection.
Baculovirus expression in insect cells represents a robust method for producing recombinant glycoproteins [4, 5]. Baculovirus-produced proteins are currently under study as therapeutic cancer vaccines with several immunologic advantages over proteins derived from mammalian sources [6]. They have a restricted range of hosts that they can infect that is typically restricted to a limited number of closely related insect species. Because baculoviruses are not harmful to humans, they are considered a safe option for use in research applications.
Baculoviruses are widely used to express heterologous genes in insect cells cultured. The baculovirus expression vector system is particularly advantageous for many application field and specialized media, transfection reagents, and vectors have been developed in response to recent advances in insect cell culture and molecular biology methods [7]. Since 1983, baculovirus system is one of the most powerful eukaryotic vector systems for recombinant protein expression [7]. Baculovirus system has significant benefits in view of safety, large-scale, and high level of gene expression.
Recently, specific proteins, called PTDs, have been identified as carriers for the efficient delivery of proteins that do not permeate living cells [8]. Although the mechanism is unknown, transduction occurs in receptorand transporter-independent manners, which appears to target the lipid bilayer directly [9]. PTDs include the peptides derived from the basic domain of HIV-1 Tat, the homeo domain of Drosophila Antennapedia and the HSV VP22 transcription factor. The short Tat peptide, YGRKKRRQRRR (residues 47-57) is sufficient for the intracellular transduction and subcellular localization [10, 11]. This domain can deliver a wide variety of proteins, ranging in size from 15 to 120 kDa, across the plasma membrane by a mechanism referred to as protein transduction [10].
In this study, we constructed a recombinant baculovirus vector system and compared efficacy of gene transfer and expression in cells and murine tissues.
The insect cell line, Sf9, was grown in Grace's medium (Invitrogen, USA), supplemented with 10% fetal bovine serum (FBS; HyClone, USA). The human hepatoma cell line, Huh7, the human cervical carcinoma line, HeLa, and the human glioblastoma, A172, were grown in Dulbecco's modified Eagle medium (DMEM; Gibco BRL, USA), supplemented with 10% FBS and 4 mM glutamine. The cultures were maintained at 37℃ in a humidified atmosphere of 95% air/ 5% CO2[8].
2.2. Construction of vector expressing fusion protein
In order to construct the plasmid with the HIV-1 Tat fused to the C-terminus of EGFP (pEGFP-Tat), two oligonucleotides were synthesized and annealed to generate a double stranded oligonucleotide encoding the 11 amino acids from the basic domain of the HIV-1 Tat. The sequences were (top strand) 5'-GATCTAGAAGGCGACAGAGGCGAAGAAGGACGGTATTAACT-3' and (bottom strand)5'-ATCTTCGTCGCTGTCTCCGTCTTCCTGCCATAATTGACAGCT-3'[8]. The double stranded oligonucleotide was inserted into pCR 2.1 (Invitrogen, USA) in order to generate pCR 2.1-Tat. Second, the EGFP gene sequence was amplified using PCR from pEGFP-N1 (Invitrogen, USA), with the sense primer 5'-AGAATCCGCTAGCGCTACCGGTCGCCCCATGG-3' and antisense primer 5'-GAAGATCTCTTACAGCTCGTCCAT-3' [8]. The
2.3. Transduction of the fusion protein into cells
When the cells were 70% confluent, the culture medium was replaced with fresh medium containing 10% FBS, and the EGFP or EGFP-Tat added to the growth medium, to a final concentration of 0.5 μM. The cells were then sampled at the times shown or after at least 10 min.
The cultured cells were grown on glass coverslips, and then infected with the recombinant baculovirus (m.o.i. 10) for 1 h. Forty eight hour after infection, the cells were washed three times with 1 ml of PBS (pH 6.4), sealed in GelMount (Biomedia, USA), then viewed on an fluorescencemicroscope (Carl Zeiss, Germany) and recorded with an Axio camera (Carl Zeiss, Germany).
The Sf9 cells were infected with Bac-EGFP, Bac-EGFP-Tat m.o.i. (multiplicity ofinfection) 10, in 6-well plates. After 48 h, the cells were lysed in a Laemmli buffer (125 mM Tris, 2% sodium dodecyl sulfate [SDS], 5% 2-mercaptoethanol, 10% glycerol and 0.001% bromophenol blue, pH 6.8) and heated to 100℃ for 5 min prior to electrophoresis. All the samples were run on SDS-10% polyacrylamide gel electrophoresis. The VSV-G-specific monoclonal antibody was obtained from Roche Molecular Biochemicals. The presence of the VSV-G protein was detected using an ECL Western blotting analysis system (Amersham Bioscience, Sweden).
We constructed novel recombinant baculovirus vector system. A peptide (RKKRRQRRR), derived from the HIV-1 Tat basic domain fused to the C-terminus of EGFP as recombinant fusion proteins, was produced to facilitate the visualization of the PTDs in cell cultures. A control EGFP expression vector was also constructed by inserting the coding sequence for EGFP into the pET15b expression vector.
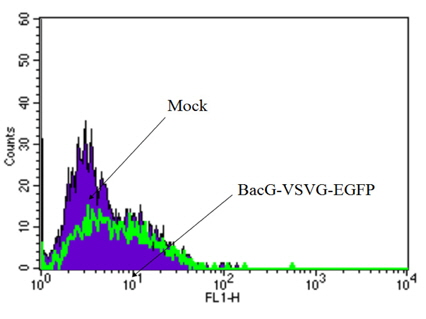
We compared through analysis of FACS of mock and HFF infected by recombinant baculovirus vector cotaining vesicular stomatitis virus G, VSVG, and EGFP in Fig. 1. Flow cytometry was commonly used to qualify and quantify the cellular import of proteins of recombinant baculovirus vector. A high transduction efficacy of were also confirmed using flow cytometry.
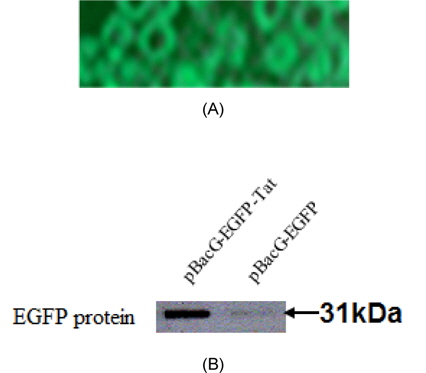
We compared expression of VSVG of recombinant baculovirus cotaining Tat and without Tat (A: EGFP expressed by VSVG using flourecense microscope, B: EGFP expressed by each vector using Western blot hybridization) in Fig. 2. The fusion proteins containing recombinant baculoviral vector were monitored during expression of the entire protein due to green fluorescence (Fig. 2A). EGFP protein of recombinanat baculovirus containing pBacG-EGFP-Tat showed higher efficacy of expression than that of recombinanat baculovirus containing pBacG-EGFP (without Tat).
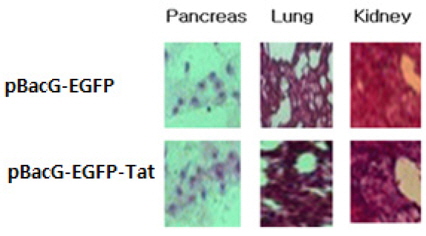
It represented that expressed EGFP of pBac-VSVGEGFP- Tat was higher than that of pBac-EGFP in murine pancreas, lung, and kidney tissues, respectively (Fig. 3). This mean transduction and expression efficiency of recombinant baculovirus containing pBacG-EGFP-Tat was higher than that of pBacG-EGFP (without Tat) when these was transfected into animal tissues.
From this results, we confirmed this novel recombinant baculovirus vector system was superior to mock or other control vector system.