Two monstrilloid copepod species belonging to the genus Monstrilla Dana are newly recorded from Korea: M. grandis Giesbrecht, 1891 and M. hamatapex
Monstrilloid copepods are basically endoparasites of marine invertebrates during their juvenile stages, but adults lacking mouthparts are free-swimming and non-feeding (Suárez-Morales, 2011; see Suárez-Morales et al., 2014 for detailed life history). The extremely reduced mouth field in non-feeding adults and the overwhelmingly constant segmentation and invariable seta/spine armature of thoracic legs 1-4 are major obstacles in monstrilloid taxonomy, which have been employed as the most discriminative and decisive characters in the taxonomy of other copepod orders. Furthermore, only a single sex has been recognized and described for most of the known species, whereas both males and females are known in ‘only reduced number of species, which makes it difficult to link both sexes’ (Suárez-Morales, 2000). In consequence, many species may have been mistakenly recognized as different species, while, conversely, a certain nominal species may actually represent a species complex including a few or several distinct species. Although the generic nomenclature is still somewhat unsettled (Boxshall and Halsey, 2004; Walter and Boxshall, 2014), about 127 species of five valid genera are currently recognized in the single family Monstrillidae Dana, 1849 (Suárez-Morales, 2011; Suárez-Morales and McKinnon, 2014). Among these genera, the major one is
In Korea, taxonomic study of monstrilloid copepods is still nearly lacking. Since the 1990s, the author has tried to collect monstrilloid copepods around the coasts of South Korea and stored in the Department of Biological Science, Daegu University, but the collection data were sporadic and sometimes incomplete, and the specimens were inadequately preserved. Recently, I resumed survey for monstrilloids by using a light trap, and intensively gathered lots of specimens. From this material, a new species,
The material examined in the present study was collected using a light trap installed at quays and wharves overnight. The trap consisted of a PVC pipe (15 cm in diameter, 42 cm long) and a couple of flash lights for SCUBA divers hanging inside the pipe. Samples were filtered at dawn through a conical plankton net or a hand net, both with 64 μm mesh, and copepods were fixed in 4% buffered formalin or in approximately 90% ethanol in the field. Methods for preparation in the laboratory, microscopic observation, and measurements were the same as in the previous paper dealing with Korean monstrilloids (Chang, 2012).
Voucher specimens for
General terminology for monstrilloid taxonomy follows Huys and Boxshall (1991), and the nomenclature for the monstrilloid antennular setation proposed by Grygier and Ohtsuka (1995) is adopted herein.
Order Monstrilloida Sars, 1903 Family Monstrillidae Dana, 1849 Genus Monstrilla Dana, 1849
Material examined. Korea: 1♀, Gangwon-do, Donghae, Mukho Port, 13 Oct 2012, Chang CY, Son YJ; 3♀♀ (1 ovi.), Gyeongsangbuk-do: Pohang, Guryongpo, Samjeong Beach, 10 Dec 2008, Chang CY, Lee J; 3♀♀, Gyeongju, Gampoeup, 16 Nov 2008, Chang CY, Lee J; 1♀, Pohang, Youngil Bay, 11 Oct 2012, Chang CY, Son YJ; 6♀♀, Ulsan, Mipo Bay, 6 Feb 1999, Chang CY, Lee J; 13♀♀, Jeollanam-do: Namhae-gun, Samdong-myeon, Eunjeom-ri, Namhae Island, 19 Oct 2012, Hong SS; 1♀, Namhae-gun, Seosang-ri, Seo-myeon, Namhae Island, 20 Oct 2012, Hong SS; 11♀♀, Wando-gun, Jung-ri, Bogil Island, 2 May 1996, Chang CY; 9♀♀, Jeollabuk-do, Buan-gun, Byeonsan-myeon, Wangpo, 2 Aug 1998, Chang CY, Lee J.
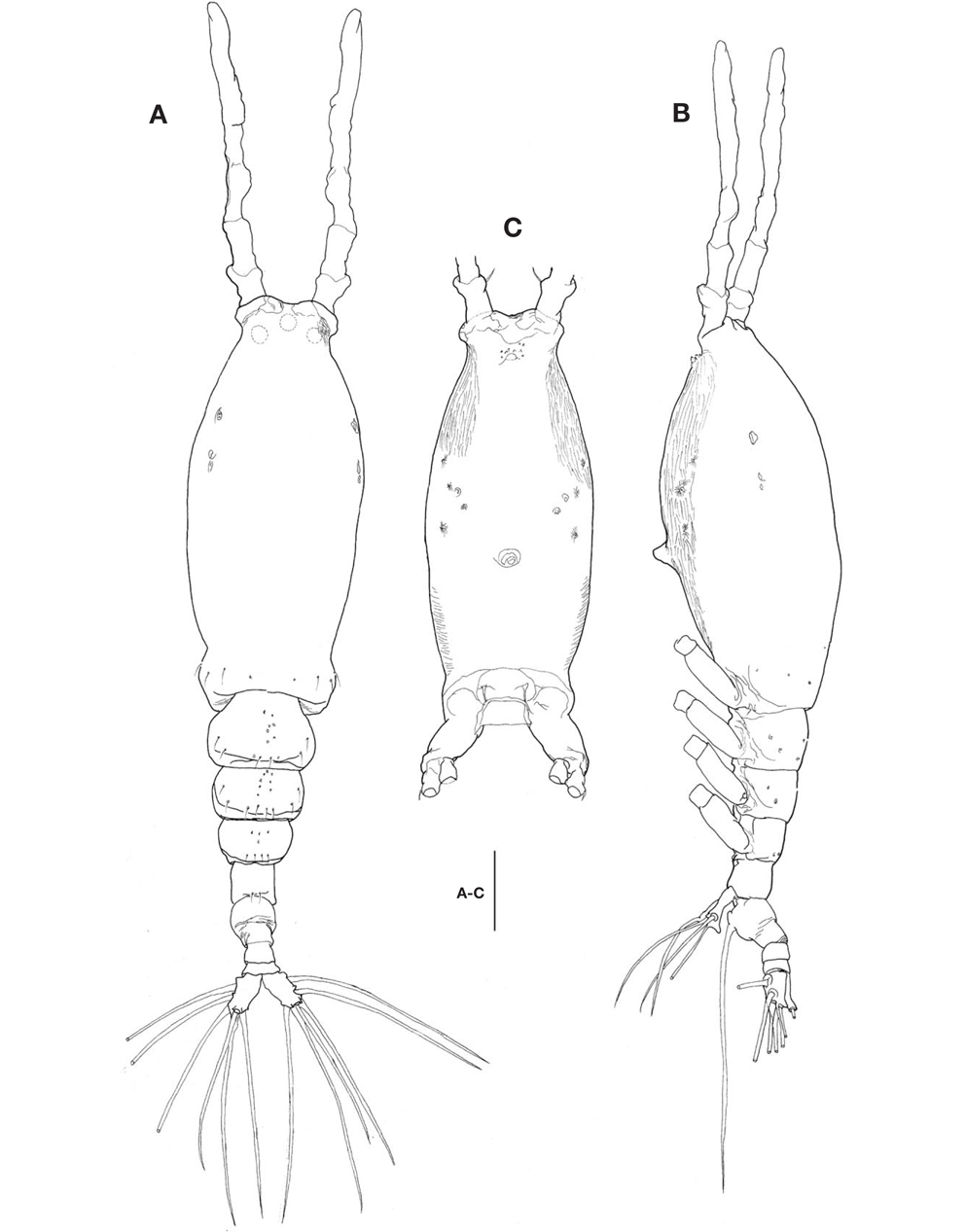
Description. Female: Body (Fig. 1A, B) rather slender and large, 1,150-1,690 μm long (mean 1,420 μm, standard deviation 274, n=8), measured from anterior end of cephalothorax to posterior margin of caudal rami, excluding antennules and caudal setae.
Cephalothorax (Fig. 1A-C) incorporating first pedigerous somite, somewhat large and relatively long, accounting for about 58% of total body length; anterior two-fifths swollen laterally and ventrally. Forehead slightly concave medially in dorsal view; anterior part just behind antennular bases abruptly curved inward. Anteriormost part of ventral surface with small, round protuberance furnished with 8-10 minute denticles (Fig. 1B, C). All three cups of nauplius eye round, not well developed (Fig. 1A). Weak, fine longitudinal wrinkles running behind antennular bases on each side of lateroventral surfaces, flanked by 3 pairs of small nipple-like scars and 2 pairs of sensory pores, ahead of oral papilla. Oral papilla (Fig. 1B, C) situated slightly posterior to midlength of cephalothorax (52.4%), slightly protruding midventrally.
Urosome (Fig. 1A, B) consisting of 4 urosomites, viz., fifth pedigerous somite, genital double-somite, third urosomite, and anal somite, followed by caudal rami, altogether accounting for 21.7% of total body length, excluding caudal setae. Genital double-somite slightly swollen laterally, bearing paired, long ovigerous spines, inserted on middle of ventral surface, basally separated, representing 37.5% of total body length, nearly twice as long as urosome, with tips pointed, not swollen (Figs. 1B, 2D), extending far beyond tips of caudal setae. Anal somite trapezoidal; lacking wrinkles or striae both on dorsal and ventral surfaces; lateral margin nearly smooth, without apparent notch.
Caudal rami (Figs. 1A, 2D) a little divergent, about 1.8 times longer than wide, with small cuticular protuberance at inner distal corner of ramus and anterior to bases of lateral caudal setae; armed with 6 well-developed caudal setae, of which 3 distal, 2 lateral, and 1 dorsal.
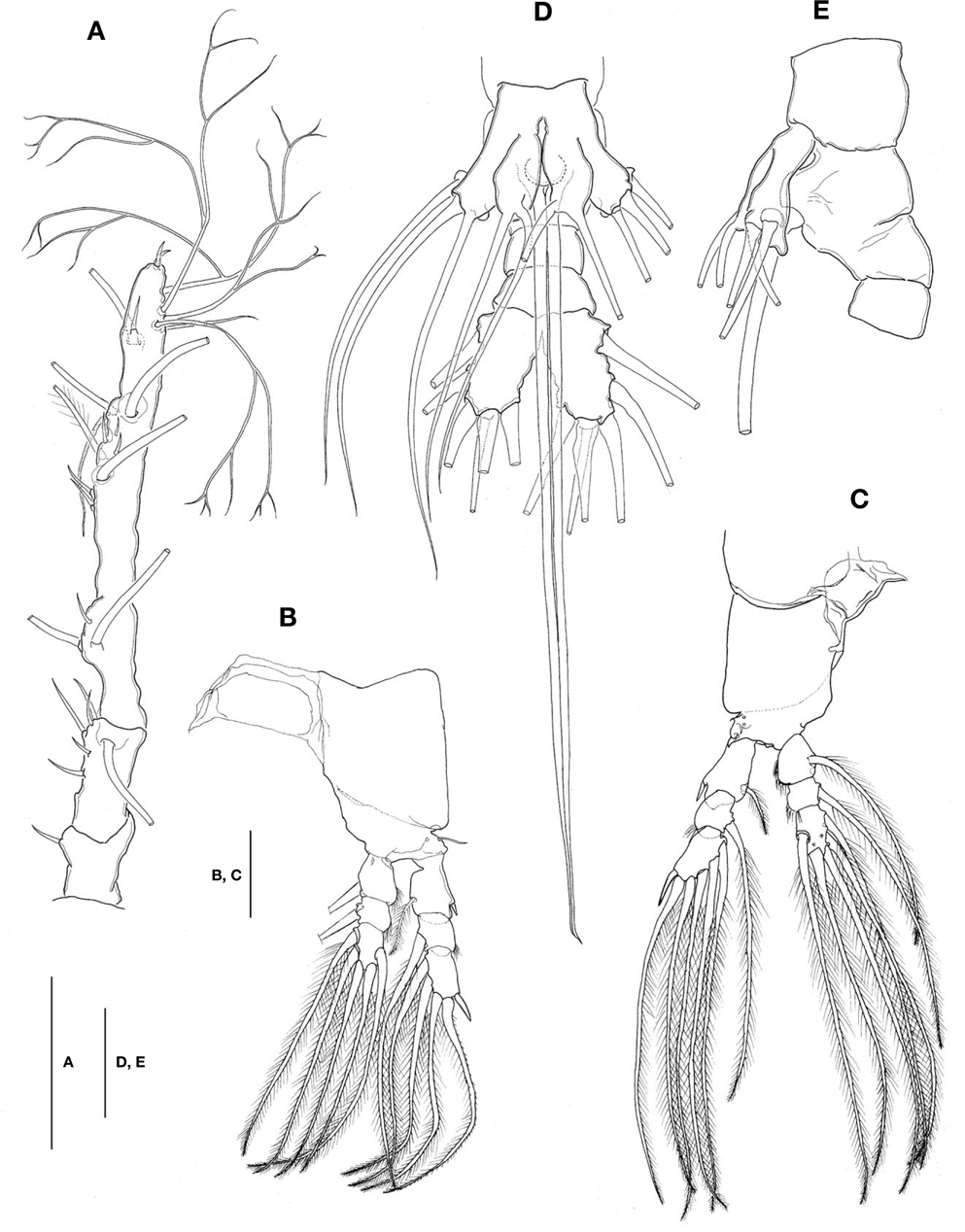
Antennule (Figs. 1A, B, 2A) long and slender, slightly longer than 48% of total body length, about 73% as long as cephalothorax; 3-segmented (ratio of lengths of segments from proximal to distal 12.4 : 17.2 : 70.4=100), with distal segment barely showing traces of fusion of three ancestral segments (purported segments 3-5). Setal armature showing standard arrangement up through purported segment 4: segment 1 with short, spinous seta 1; segment 2 with 5 spinous setae (2d1-3, 2v1-2) medially and 1 long, plumose seta dorsally (IId); purported segment 3 with short, spinous seta 3 and 2 long, plumose setae both ventrally and dorsally (IIIv, IIId); purported segment 4 with 5 spinous setae (4v1-3, 4d1-2), 2 plumose setae (IVd, IVv), 1 long aesthetasc (4aes). Purported segment 5 with spinous seta 5 medially, 3 plumose setae (Vd, Vm, Vv), setae b1-4 dichotomously branched from proximal quarter or third, and b5 slender, short, not branched; b6, 6aes absent. Distal 2 claw-like spines (61 and 62) slightly different in length, 62 about 1.2 times longer than 61.
Legs 1-4 all with both endopod and exopod 3-segmented. Coxa of legs 1-4 lacking seta; intercoxal sclerite with smooth posterior margin; both anterior and posterior surfaces smooth without transverse row of spinules or setules. Coxa and basis not markedly divided. Basis lacking seta distomedially; outer seta slender, naked, except for that on leg 3, which is much longer and setulate. First and second endopodal segments of legs 1-4 slightly swollen laterally with hairs along outer distal margin. Outer distal spines on first and third exopodal segments of legs 1-4 feeble, much shorter than segments bearing them (Fig. 2B, C). Seta/Spine armature on swimming legs 1-4 as follows (Roman numerals indicate number of spines, and Arabic numerals indicate number of setae):
Leg 5 (Fig. 2D, E) bilobed, both members of pair fused medially. Outer lobe elongate, lateral margin curved outward, armed with 3 long, plumose setae; thumb-like process present on distal part of inner margin of outer lobe (Fig. 2E). Inner lobe oblong, about 2 times as long as wide, its tip not quite reaching distal end of outer lobe, furnished with 2 apical setae of subequal length.
Male: Not collected.
Remarks. This species was originally and briefly diagnosed by Giesbrecht in 1891 from material collected off southern Patagonia in the southeastern Atlantic Ocean (49°S, 65°W) (Grygier, 1995). Later, Giesbrecht (1893) provided a more detailed, but still rather inadequate description and illustrations of males and females, based on this material. Neither his text nor his illustrations note the presence of a thumb-like or knob-like process on the outer lobe of leg 5 in the female. The present Korean specimens share it with Shen and Bai’s (1956) Chinese specimens, Isaac’s (1974a) and Huys and Boxshall’s (1991) British specimens, and Suárez-Morales’ (2000) specimens from Toulon Bay in the northwestern Mediterranean Sea. Isaac (1974a) mentioned another difference between his material and Giesbrecht’s (1893) figure: the two apical setae on the inner lobe of leg 5 were nearly equal in length in Isaac’s specimens, but the inner seta was much shorter than the outer in Giesbrecht’s figure. All the Korean specimens examined, as well as the other records above, agree with Isaac’s description. Dr. Grygier (personal communication) provided a very significant suggestion about this, as follow: “Taking these facts into account, as well as biogeographical considerations, it seems unlikely that the original material of
The relative length of the ovigerous spines is rather consistent in the Korean specimens, slightly less than twice as long as the urosome, which agrees well with Huys and Boxshall (1991) and Suárez-Morales (2000).
A long and slender antennule, equal to about half of the total body length, is a characteristic of this species. Suárez- Morales (2000) provided an excellent illustration of the antennules and analyzed the setation pattern in detail for the first time, using Grygier and Ohtsuka’s (1995) nomenclature. The setal armature of the present Korean female specimens coincides with that of Suárez-Morales’ (2000) Mediterranean specimens, except that the seta b5 on last segment is lacking in Suárez-Morales (2000) specimen, while the weakly-branched seta b5 is present in all the Korean specimens examined (cf. Fig. 2A). Furthermore, the relative length of the antennules shows a little variability: slightly less than half the total body length in the Korean specimens and the British specimens of Huys and Boxshall (1991, fig. 2.5.1), but slightly longer than 53% of the total body length in Suárez-Morales’ (2000) specimens.
Distribution. Northeast Atlantic from Britain to Morocco, Mediterranean and Black Seas, Suez Canal, West Indies (Barbados, Puerto Rico), Brazil, Argentina, Chile, China (Bohai Bay, Chefoo, South China Sea), Vietnam, Japan (Hokkaido, Shimonoseki), Korea (Yellow Sea, South Sea, East Sea).
Material examined. Korea: 4♀♀ (1 ovi.), Gyeonsangbukdo: Yeongdeok-gun, Ganggu-myeon, Geumjin-2-ri, 27 Jul 2007, Chang CY, Lee J; 4♀♀, Pohang-si, Songra-myeon, Hwajin-1-ri, 17 Aug 2010, Chang CY, Yoo JG; 7♀♀, Pohang-si, Youngil Bay, 11 Oct 2012, Chang CY, Son YJ; 2 ♀♀, Pohang-si, Yangpo-ri, 30 Jun 2012, Chang CY, Son YJ; 52♀♀ (3♀♀- NIBRIV0000277644), Pohang-si, Janggi-myeon, Kyewon-ri, 15 Sep 2012, Chang CY, Son YJ; 2 ♀♀, Pohang-si, Janggi-myeon, Duwon-ri, 3 Nov 2011, Chang CY; 3♀♀ (NIBRIV0000277645), same locality, 15 Sep 2012, Chang CY, Son YJ; 1♀, Busan, Gijang-eup, Wolnae Bay, 13 Nov 2012, Chang CY, Son YJ; 1♀, Busan, Jagalchi, 10 Nov 2012, Chang CY, Son YJ; 1♀, Gyeonsangnam-do, Sangju-ri, Namhae Island, 1 Jul 1998, Chang CY, Lee J.
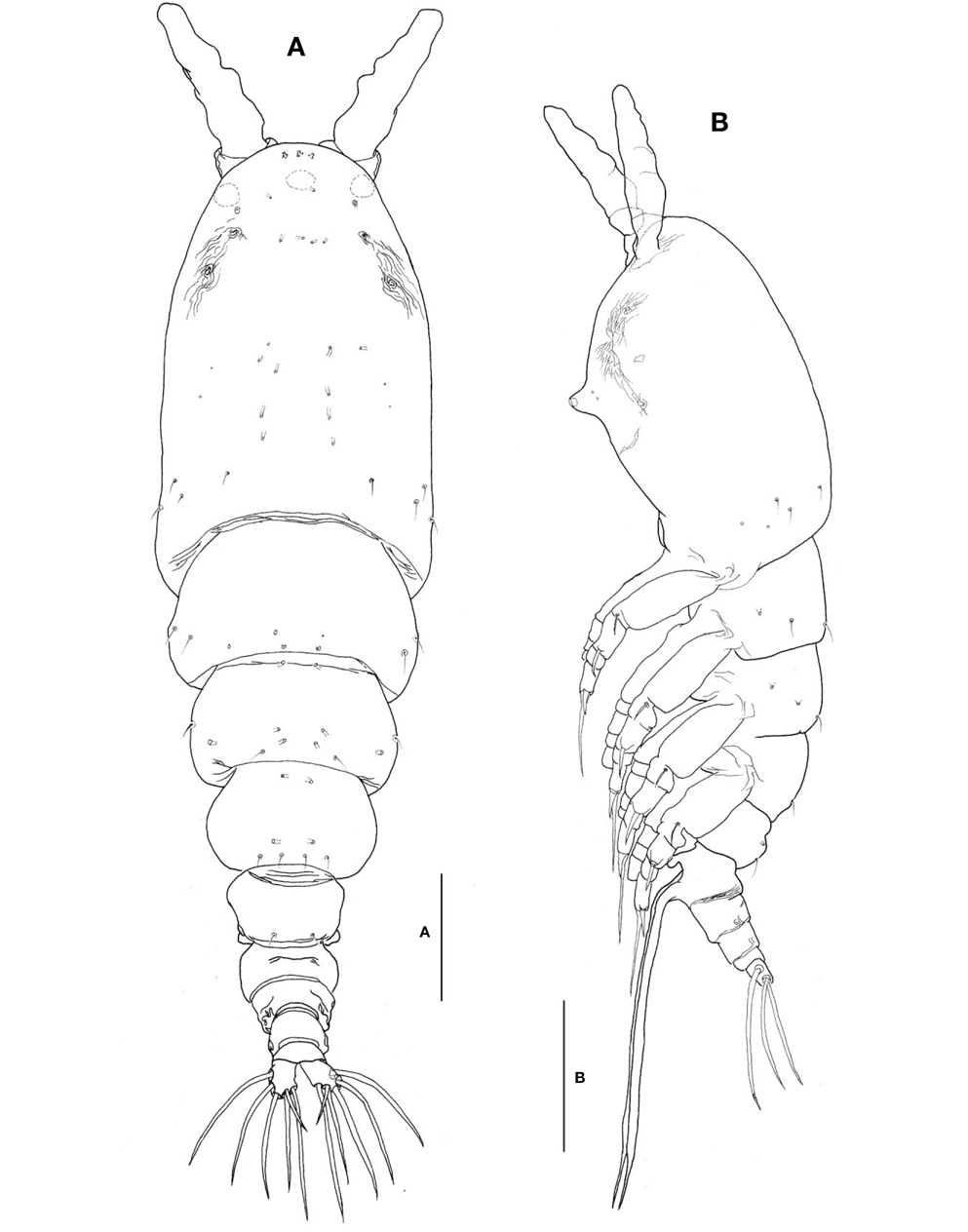
Description. Female: Body (Fig. 3A, B) large, relatively stubby; 1,321-1,642 μm long (mean 1476.2 μm, standard deviation 153, n=12), measured from anterior end of cephalothorax to posterior margin of caudal rami, excluding antennules and caudal setae; widest at posterior margin of cephalothorax, mean of length to width ratio 0.29, then tapering regularly posteriorly. Prosome comprising cephalothorax and 3 thoracic somites, each bearing pair of biramous swimming legs. Cephalothorax, incorporating first pedigerous somite, bulletshaped, large (mean 667 μm long), accounting for about 45% of body length; anterior third slightly swollen laterally; dorsal surface ornamented with several sensory pores on anterior end of cephalothorax, flanking 2 pairs of large pores posteriorly, then with 1 transverse row of 4 small pores at level of anterior quarter of cephalothorax; 4 pairs of small pores situated middorsally just behind midlength of cephalothorax, these being furnished with subcuticular ducts; 4-5 pairs of sensilla present dorsolaterally near posterior margin of cephalothorax.
Nauplius eye consisting of 1 anteroventral and 2 lateral small cups, widely separated from each other in dorsal view (Fig. 3A). Longitudinal wrinkles present behind antennular bases on each side of ventral surface, flanked by paired nipple- like scars (Fig. 3B). Oral papilla (Fig. 3B) situated slightly anterior to midlength of cephalothorax (42.4%), slightly protruding ventrally. Weak wrinkles present around oral papilla and nipple-like scars.
Urosome (Figs. 3A, B, 4A) consisting of fifth pedigerous somite, genital double-somite, third urosomite, and anal somite with caudal rami, accounting for about 29% of body length, excluding caudal setae. Genital double-somite slightly produced outer posteriorly, with transverse wrinkles along posterior margin dorsally (Fig. 3A, B). Genital double-somite bearing paired, long ovigerous spines, inserted on middle of ventral surface, basally separated, representing about 43% of total body length, nearly 1.5 times longer than urosome, with tips pointed, not swollen (Fig. 3B), extending far beyond tips of caudal setae. Anal somite (Fig. 4A) trapezoidal; lacking wrinkles or striae both on dorsal and ventral surfaces; lateral margin nearly smooth, without apparent notch.
Caudal rami (Fig. 4A) a little divergent; ramus suboval, with nearly straight inner margin, about 1.3 times longer than wide; furnished with 5 long, plumose setae, subequal in length and breadth, and 1 slender, short, naked seta dorsal to second medial seta.
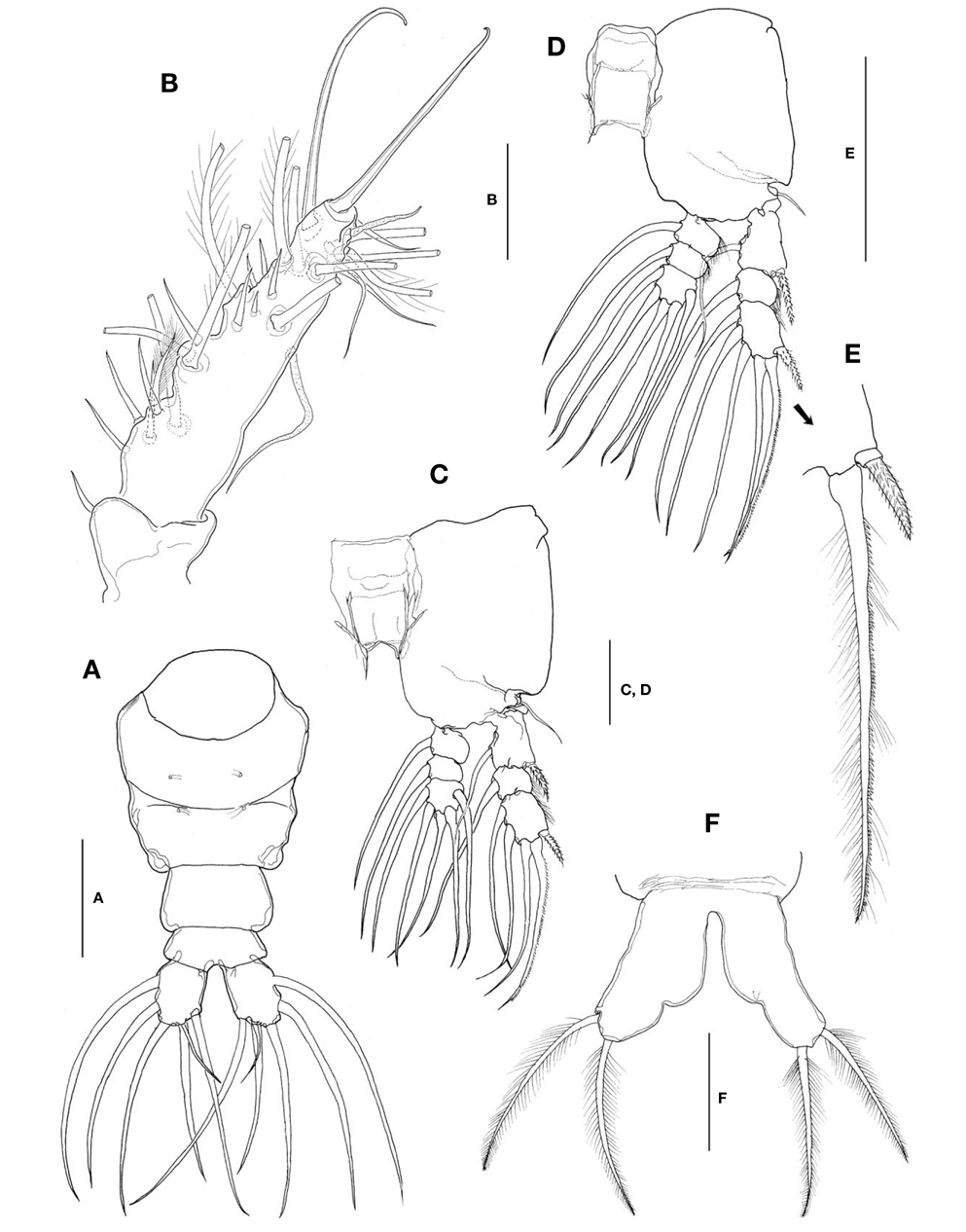
Antennules (Fig. 4B) short and relatively stubby, about 19% of total body length, and about 43% as long as cephalothorax; 2-segmented, consisting of short, basal segment and long, compound distal segment. Basal segment bearing 1 short, naked seta (seta 1) anterodistally. Distal compound segment not clearly defined, with 5 indented parts, possibly representing original segments 2-6, showing standard arrangement of long, plumose setae, 1-2-2-3-0 from proximal to distal. Short, spiniform setae arranged normally in 1-5-1-5-1-2 pattern; seta 4v2 not doubled, much larger than 4v1. Two aesthetascs (long 4aes and short 6aes) present as usual. Six outer distal b-setae not branched. Distal 5-seta and apical 62-seta modified to form long, hook-like spines. Apical 2 chela-like spines (61 and 62) differing in length by 2.4-2.7 times.
Legs 1-4 all with both endopod and exopod 3-segmented. Coxa of legs 1-4 lacking seta; intercoxal sclerite subrectangular, with smooth posterior margin slightly produced laterodistally; both anterior and posterior surfaces smooth without transverse row of spinules or setules. Coxa and basis not fully divided. Basis lacking seta distomedially; outer seta slender, naked, of similar length in all 4 limbs. First and second endopodal segments of legs 1-4 slightly swollen laterally with hairs along lateral margin. Outer distal seta on third exopodal segment spiniform, with heterogeneous ornamentation along outer and inner margins; outer distal spines on first and third exopodal segments of legs 1-4 feeble, slightly shorter than segments bearing them, ornamented with minute secondary setules (Fig. 4E).
Seta/Spine armature of swimming legs 1-4 as follows (Roman numerals indicate number of spines, and Arabic numerals indicate number of setae):
Leg 5 (Fig. 4F) single-lobed, medial margin with slight swelling representing vestige of endopodal lobe; exopod armed with 2 plumose apical and subapical setae, subequal in length.
Male: Unrecognized.
Remarks. This species was originally reported as “
This species is evidently the most common and frequent monstrilloid species in South Korea, and it occurs all year round.
Distribution. Japan (Tanabe Bay and Ago Bay, Honshu), Korea (East Sea, South Sea).