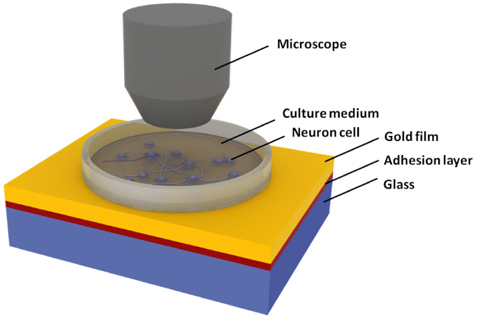
Extracellular measurement of neural activity is a key technology for research on neural interfaces such as cultured neural networks. While electrical recording based on microelectrode arrays has been widely used, an electrical method often suffers from stimulation artifacts when an electrical signal is applied [1]. Moreover, the electrodes should be placed close to the neuronal cell body for increasing the efficacy of the interface and excluding the noise sources. On the other hand, as demonstrated lately [2], alternative optical approaches have drawn tremendous interest due to their advantages such as noninvasive and artifact-free monitoring and high spatial resolution. Although voltagesensitive fluorescence dyes allow direct visualization of a membrane potential change, optical staining has fundamental drawbacks of cell toxicity, a time-consuming labeling process, and photobleaching [3]. In recent years, a surface plasmon resonance (SPR) technique has been introduced as intrinsic and label-free optical recording method.
In principle, an SPR biosensor measures a refractive index change at a dielectric-metal interface. As SPR detection is rapid, quantitative, and sensitive, it has been utilized to monitor the kinetics of surface-immobilized interactions [4]. Those properties allow the SPR system to sense the neural activities because action potential is accompanied by a small change in membrane-localized refractive index. In the previous studies, we verified the effectiveness of SPR-based optical detection in both in vitro and in vivo neural recordings for isolated rat sciatic nerve and somatosensory cortex [5,6]. However, the following key issues should be addressed for practical use of SPR-based neural recording: 1) What are requirements of the glass substrate to provide better performance and to ensure biocompatibility in SPR sensing? We demonstrate numerically whether glass material of a high refractive index is useful as an SPR substrate in terms of sensitivity and dynamic range in measuring neural signals [7]. In particular, a leaded flint glass cannot assure the biosafety of culture environments even though the neuron cells are not directly attached to the toxic glass, but separated from it via a gold film and an adhesion layer. 2) What are the effects of thin metallic films on the cell viability? Gold is usually biocompatible and inert, but an exposure to a titanium or chromium adhesion layer might be injurious to living cells.
Among various instrumental SPR sensing platforms, the most widely used substrate includes BK7 glass, which is known as a biocompatible crown glass. Crown glass has a low refractive index and is produced from alkali-lime silicates containing potassium oxide. On the other hand, flint glass has a relatively high refractive index ranging from 1.46 to 2.00. To obtain a higher refractive index, several additives such as lead oxide, titanium oxide, or zirconium oxide are usually contained in the flint glass. In terms of cytotoxicity, lead-based glass substrates and adhesion metal layers may have harmful effects on the cultured cells [8, 9]. In other words, while the neurons are cultured onto a gold film, the stack configuration of SPR templates including toxic materials can exert unidentified adverse effects on the neuron cells.
In this study, we, for the first time to our knowledge, investigate whether the viability of cultured neuron cells can be affected by the substrate materials. To evaluate the cellular cytotoxicity in a quantitative way, we count the number of primary hippocampal neurons grown on various SPR substrates, fabricated by combining three different glasses of BK7, SF10, and NSF10 with two adhesion metal films of chromium and titanium. It is expected that our study will serve as the first step to design an optimal configuration of the SPR substrate for improving the biocompatibility of an in vitro neural recording system.
2.1. Preparation of SPR Substrates
Three kinds of glasses were purchased from Marienfeld GmbH (Lauda-Königshofen, Germany) for BK7, Korea Electro- Optics (Bucheon, Korea) for SF10, and Schott (Mainz, Germany) for NSF10. While the Marienfeld BK7 glass containing 10% boric oxide has been frequently used as a substrate for cell cultures due to low alkali content, there have been few reports on the application of flint glasses such as SF10 and NSF10 to in vitro studies on live cells. Refractive indices of the glasses are, respectively,
Primary hippocampal neurons were obtained by referring to Ref. [10]. Whole brains were isolated from fetuses of embryonic day 17 Sprague-Dawley rats (Samtako, Osan, Korea) and were placed in sterile buffered saline solution (BSS). Hippocampi were dissected from the separated cerebral hemispheres, placed in ice-cold BSS and incubated in 0.25% trypsin (Sigma, St. Louis, MO, USA) for 15 min at 37℃. Digested hippocampi were dissociated three times for 5 min in BSS at room temperature by triturating with fire-polished glass pipettes. Neurons were seeded at the density of about 300 cells/mm2 on the SPR samples in minimal essential medium (MEM) supplemented with 10% horse serum and 0.1% pyruvic acid (Invitrogen, Carlsbad, CA). After 4 hr incubation, the MEM medium was replaced with serum-free Neurobasal media (Invitrogen, Carlsbad, CA, USA) supplemented with B27 (Invitrogen) and Gluta- MAX (Invitrogen). During the experiments, the media was replaced with fresh every 3 days. Cultures were maintained at 37℃ in a 5% CO2, 95% air humidified atmosphere.
2.3. Cell Counting and Data Analysis
A hemacytometer was used to obtain the initial neuronal cell density of 300 cells/mm2. After seeding neurons on the surface, the cells were imaged using an inverted microscope (IX71, Olympus, Japan) for 3 weeks. Only the cell bodies with axons and dendrites were counted as the number of cells during in vitro cell culture. The number of cells per unit area of 440 μm × 273 μm was counted 3 times in different sites for each sample after replacement of the media which were replaced every 3 days.
2.4. Transfer-matrix Method (TMM)
The analytical solution of reflectance
where
Here,
where
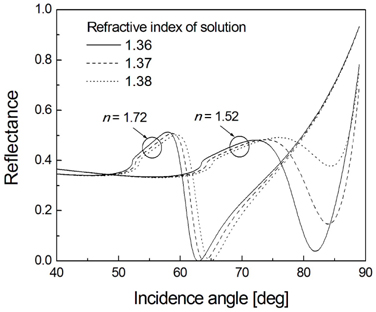
First of all, we numerically demonstrate whether the SPR sensing performance for neural recording can be improved by a high refractive index glass, when compared against a conventional crown glass. Using the TMM, we determine the reflectance characteristics of a 4-layer SPR system consisting of glass substrate, chromium adhesion layer, gold film, and cell culture medium [12]. Based on the fact that an effective refractive index of a single cell is known to be about 1.37 [13], as neural activities are accompanied by small changes in the cellular volume and the membrane-localized liquid phase, the refractive index of the solution is assumed to be varied in the range from 1.36 to 1.38. Mechanical fluctuations such as cell swelling and shrinkage during the period of action potential also contribute to the refractive index change. The relative permittivities ε = (
The calculated SPR curves are shown in Fig. 2 for two glass substrates of low and high refractive indices n. When a refractive index of the surrounding solution increases, clear advantages in the latter case are evident. The resonance band of a high refractive index glass is sharper and deeper, implying superiority in detection sensitivity and signal-to-noise ratio. On the other hand, the glass substrate of a low refractive index gives a broad and shallow resonance peak. In particular, a higher SPR angle over 80° makes practical implementation extremely difficult and dynamic range in measurement very narrow. As a result, the choice of a higher refractive index glass is appropriate for monitoring the neural signals if its biocompatibility is verified. Recently, a crown glass with a high refractive index, for example, LAK34, has been developed. However, owing to its low acid resistance even in weak acid, an acidic solution-based surface cleaning process to remove organic residues may cause severe damages to the glass substrate.
Hence, in the following section, we intend to demonstrate the possibility of flint glass for optical neural recording as an alternative to a crown glass. We perform a biosafety test using two different flint glasses of SF10 and NSF10 with an equal refractive index. While a flint glass generally contains lead oxide, NSF10 is a lead-free glass whose lead compound is replaced with other additives such as titanium dioxide and zirconium dioxide without altering the optical properties.
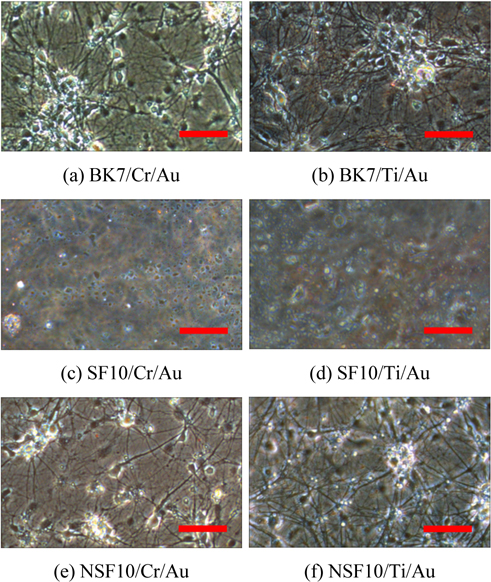
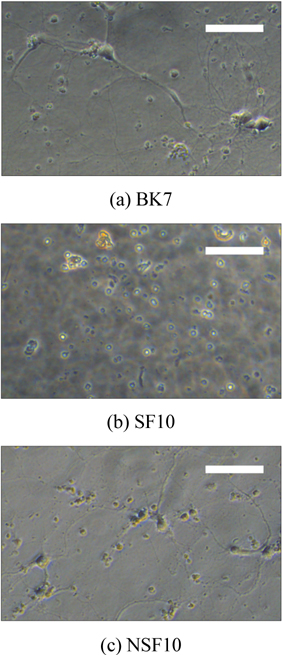
Figure 3 shows microscope images of the cultured neuron cells at 12 days in vitro (DIV). While the results are not shown, we first observed the growth and position of neurons at 3 DIV and each neuron started to make small connections with neighboring cells. At 12 DIV, while the BK7 and NSF10 glasses show greatly concentrated cell bodies and dendrites of neurons, no cellular structure is found at the SF10 glass, which implies that SF10 glass inhibits neuronal cell growth by releasing toxic materials that can inactivate neural activities. For BK7 and NSF10 glass substrates, a number of neurons are still alive after 18 DIV and dense and multilayered axons and dendrites in the cell bodies are exhibited.
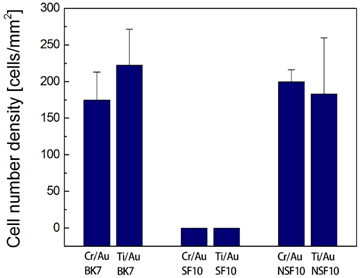
It is also interesting to note that there is no significant difference in overall trends of cell survival, cell morphology, and neurite elongation between the substrates with chromium and titanium. Adhesion layers might have no adverse influence on the metabolic activity of cultured cells because a 5-nm thick layer is too thin to affect the cell viability. The cell number densities obtained are, respectively, 175 ± 38 cells/mm2 for BK7/Cr/Au, 222 ± 49 cells/mm2 for BK7/ Ti/Au, 200 ± 17 cells/mm2 for NSF10/Cr/Au, and 183 ± 77 cells/mm2 for NSF10/Ti/Au substrate, as presented in Fig. 4. While multiple sites in a given sample are measured by spatial translation to ensure consistency and thereby to reduce variation, the standard error is relatively large because the cell distribution is not perfectly uniform on the gold substrate.
As a next set of experiments to verify that SF10 glass is harmful, neuron cells are directly cultured on a glass substrate without metal films. Cell images at 15 DIV in Fig. 5 show the cytotoxic effect caused by lead-based SF10 glass. Contrary to the cultures in BK7 and NSF10 glasses, a rapid neuronal cell death occurs after exposure to SF10 glass. It is attributed to the fact that lead causes the axons of nerve cells to degenerate and lose their myelin coats. It is also known that lead enters the neurons via voltage-sensitive calcium channels and induces mitochondrial dysfunction, which can lead to a release of cytochrome C, activation of a variety of caspases and cleavage of downstream death effector proteins, and ultimately results in apoptotic cell death [15]. In short, the existence of toxic materials in glass substrate should be avoided during the culture for improving the cell viability.
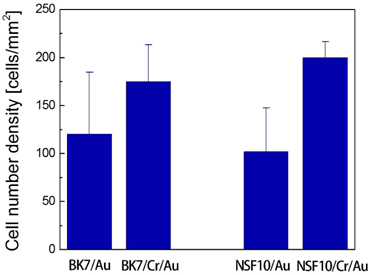
Another remaining issue of interest is the role of adhesion layers in neuronal cell culture. Thin transition metal films, such as chromium, titanium, and tungsten are generally employed as an adhesion promoter between a metal film and a glass substrate. They not only improve the adhesion, but enhance the stability of the noble metal layer. If the adhesion film is not used, the metal film supporting plasmon excitation can often be peeled off and damaged by the cleaning processes. In this experiment, we compare the cell number density of SPR substrates with 5-nm thick chromium adhesion with the cases in which a gold film is directly deposited on a glass substrate. In Fig. 6, a higher cell number is obtained in the presence of chromium for both BK7 and NSF10 glasses. At 12 DIV, the cell number densities are determined to be 120 ± 65 cells/mm2 for BK7/Au and 102 ± 46 cells/mm2 for NSF10/ Au substrates, which are approximately 50 ~ 60% of the cell survival for SPR substrates with a chromium layer. The substrate instability is responsible for poor attachment of neuron cells and this may contribute to a smaller cell density and a larger standard error. On the other hand, it has been reported in several in vitro studies that chromium thicker than 50 nm can inhibit the metabolic response of cultured cells such as stem cells [8], osteoblastic cells [9], macrophages [16]. In particular, since a thick adhesion film can degrade the performance of SPR-based neural recording system due to a large imaginary part of its complex dielectric constant, careful substrate design is required for an efficient neuron cell culture.
In this study, we demonstrate that flint glass substrates of a high refractive index have a potential for improving SPR-based neural recording system due to significant advantages in detection sensitivity, signal-to-noise ratio, and dynamic range compared to a conventional crown glass. Based on the cytotoxicity test performed to verify the possibility of applying the flint glass to in vitro neuronal cell culture, we find that SF10 glass including lead oxide shows harmful effects to the cultured neurons, whereas another lead-free flint glass of NSF10 is not problematic. The lead-mediated cytotoxicity is inevitable even when the neuron cells are separated from SF10 glass via thin adhesion layer and gold film. It is also found that the adhesion layer of chromium and titanium plays an important role in achieving the substrate stability. The cell density of surviving neurons on gold films deposited with a metallic adhesion layer was approximately twice that of the films without the adhesion layer due to the increased mechanical stability. As a result, by applying a lead-free flint glass of a high refractive index and a thin adhesion film to SPR substrate, we may expect to accomplish an improved performance in culturing the neuron cells and recording their signals.