Many rocky seashore areas of Korea and Japan are dominated by coralline algae such as Lithophyllum yessoense (Suzuki et al., 1998; Kim, 2000). However, as calcareous algae cover the surfaces of rocks in a pink-colored crust, the area covered by seaweed flora decreases. This algal whitening phenomenon is observed in barren ground, coralline flats, and deforested areas, and is associated with specific species of coralline algae (Tokuda et al., 1994). Since 1990, the area affected by algal whitening, which runs from south Cheju Island to the middle East Sea, has expanded (Chung et al., 1998). In this area, most of the fleshy seaweed has disappeared from the rocks because of algal whitening, which reduces food sources and spawning locations for fish and shellfish. This phenomenon is now considered a natural hazard adversely affecting marine ecosystems and damaging commercial fishing areas. Although biological (Agateuma et al., 1997; Daume et al., 1999) and physical (Masaki et al., 1984; Johnson and Mann, 1986) factors may be sufficient to prevent the recruitment of fleshy seaweeds, allelopathic bromoform (Ohsawa et al., 2001) and fatty acid (Kim et al., 2004; Luyen et al., 2009) substances inhibit the settlement or germination of seaweed spores. One approach to restore fleshy seaweed colonization in these areas is the removal or inhibition of living coralline algae. Before applying chemicals in the field, it is necessary to test the in vitro inhibitory activity of several calcification inhibitors against coralline algae to identify compounds suitable for coralline species inhibition in areas affected by algal whitening. Alternatively, coralline algae may be used to predevent fouling by fleshy seaweeds. When a biomimetic coralline algal material is prepared, it can be used as an environmentally friendly anti-fouling material. To generate biomimetic coralline material, it is necessary to add a calcification inhibitor to the material to prevent additional attachment and the blooming of living coralline algae on the product. Thus, a calcification inhibitor can be used to remediate algal whitening and prepare a biomimetic coralline material. In this report, inhibitors were quantitatively screened using a triphenyltetrazolium chloride (TTC) assay.
The coralline algae L. yessoense and Corallina pilulifera were collected from the rocky intertidal area at Cheongsapo (35˚09ʹ28ʺ N, 129˚11ʹ47ʺ E), on the east coast of Busan, Korea. Stones covered with coralline algae were transported in a container of seawater to the laboratory. After rinsing well with autoclaved seawater to remove epiphytes and debris, the encrusted or non-articulated tissues of L. yessoense were sonicated three times with 30-s pulses of an ultrasonic water bath (low-intensity frequency of 40 kHz) to remove other microepiphytes. The tissue was then scraped off the stones using a saw and thoroughly washed at least six times by centrifugation at 1,000 g for 30 s (Kang et al., 2005). Articulated coralline tissues were cleaned by brushing thoroughly and sonicating (40 kHz) twice for 1 min in autoclaved seawater, and then immersed in 1% Betadine for 2 min to eliminate epiphytes (Jin et al., 1997). The articulated coralline tissues were then rehabilitated at 18°C in Provasoli’s enriched seawater (PES) (Provasoli, 1968) for 1 day before use.
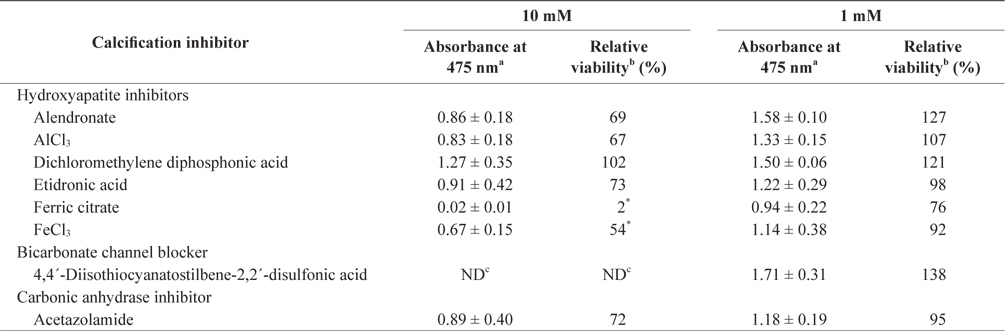
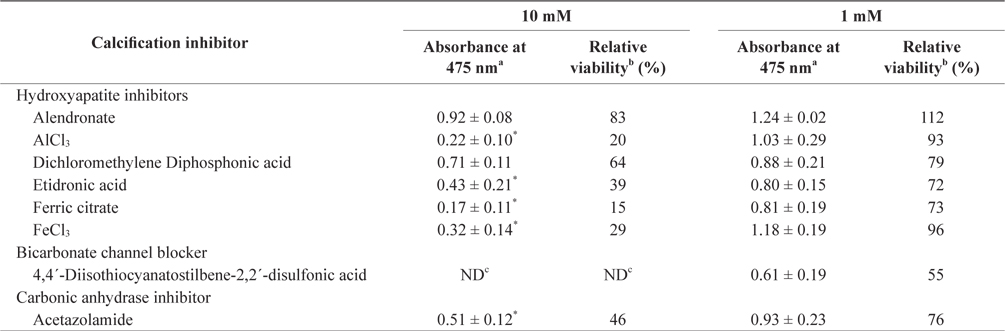
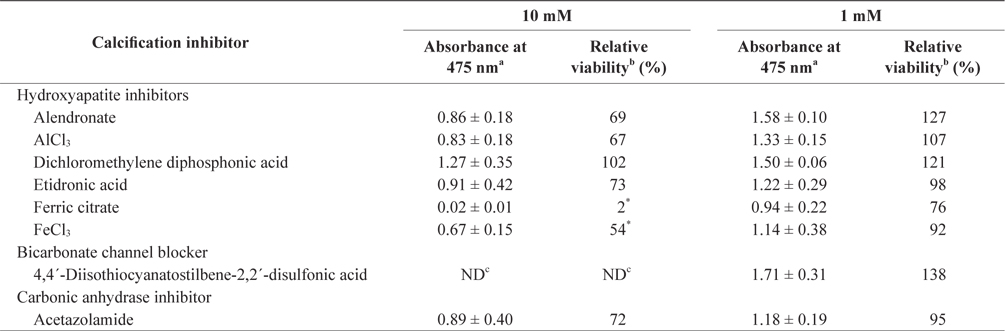
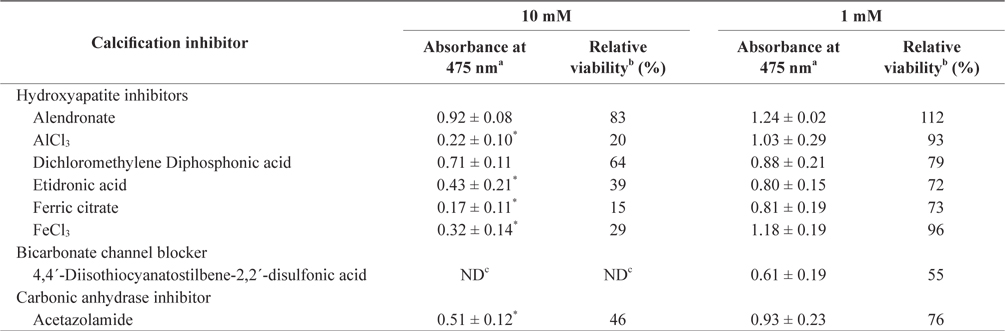
In the tissue cultures of coralline algae, six hydroxyapatite inhibitors (alendronate sodium trihydrate, AlCl3, dichloromethylene diphosphonic acid, etidronic acid, ferric citrate, and FeCl3) and one bicarbonate channel blocker (4,4´-diisothiocyanatostilbene-2,2´-disulfonic acid) as calcium inhibitors were dissolved in distilled water and added to the tissue culture solution at concentrations of 10 or 1 mM, respectively. In addition, an impermeable carbonic anhydrase inhibitor (acetazolamide) was dissolved in dimethyl sulfoxide (DMSO) and added to the solution. To measure tissue viability, 25 μL of each inhibitor was added to 5 mL of PES medium containing 0.1 g of L. yessoense or 0.05 g of C. pilulifera. The mixture was cultured for 5 days at 16°C with rotation at 20 rpm under a photon flux density (fluorescent light) of 40 μmol m-2 s-1 and on a light cycle of 16-h light/8-h dark. A reference culture was prepared by mixing 25 μL of distilled water or DMSO in the same medium. After harvesting the tissues by centrifugation at 3,000 g for 30 s, cell viability was measured using the TTC assay. The relative viability (%) was calculated as: (S/C) × 100, where S equals the absorbance of the tissue with inhibitors and C equals the absorbance of the reference culture.
The TTC assay described by Park et al. (2006) was used. A total of 1 mL of 0.8% TTC solution in seawater containing 50 mM Tris-HCl buffer (pH 8.0) was added to the tissue in a microtube and incubated in the dark for 1.5 h at 20°C under a drop of mineral oil (M-3516; Sigma, St. Louis, MO, USA). The tissue was then rinsed four times by centrifugation at 3,000 g for 30 s with sterilized seawater. Triphenylformazan (TPF) that formed in the tissues was extracted with 0.6 mL of 0.2 N NaOH in 75% ethanol by heating for 15 min at 60°C. Next, TPF was partitioned by adding 0.6 mL of hexane followed by vortexing. After centrifugation for 1 min, the amount of TPF from the top phase was quantified by measuring the absorbance at 475 nm.
For each assay with calcification inhibitors and control samples, the experiments were repeated at least three times. The mean values of the index were compared to the control using Student’s t-test.
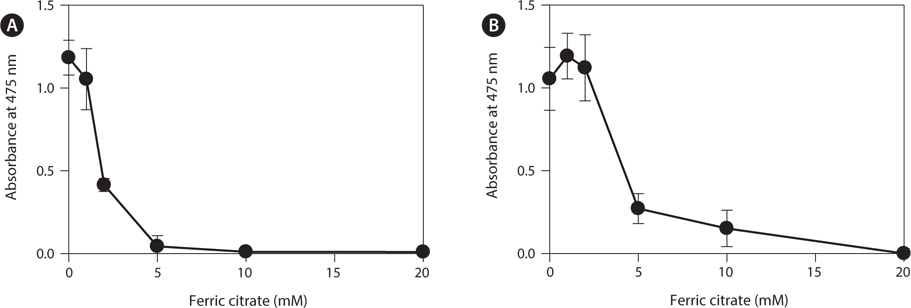
To determine the effects of the calcification inhibitors on coralline viability, we compared eight commercially available inhibitors, including six hydroxyapatite inhibitors, one bicarbonate channel blocker, and one carbonic anhydrase inhibitor. Each compound at 1 and 10 mM, respectively, was added to the coralline culture, and the viability of the culture after 5 days was measured using the TTC assay. For crustose L. yessoense tissues, reference cultures without calcification inhibitors reached an absorbance of 1.24. Ferric citrate showed an absorbance of 0.02, which corresponded to 2% relative viability at 10 mM (Table 1). At 1 mM, ferric citrate inhibited viability to 76% compared to the control. Next, FeCl2 inhibited viability to 54% at 10 mM. The effects of the calcification inhibitors on the viability of articulated coralline C. pilulifera were also determined using the TTC assay after 5 days of culture. A reference culture lacking calcification inhibitors reached an absorbance of 1.11. Among the inhibitors, ferric citrate, AlCl3, and FeCl3 inhibited viability to 15%, 20%, and 29% compared to the control, respectively, at 10 mM (Table 2). Overall, ferric citrate most significantly suppressed the viability of L. yessoense and C. pilulifera. To explore treatment concentrations, we used a dose-response curve to determine the concentration resulting in 50% inhibition (IC50) and the minimum concentration resulting in 100% inhibition (MIC). For ferric citrate against L. yessoense, a typical gradient of viability inhibition ranged sigmoidally with IC50 and MIC values of 1.7 and 10 mM, respectively (Fig. 1A). For C. pilulifera, a typical gradient of inhibition showed IC50 and MIC values of 3.8 and 20 mM, respectively (Fig. 1B).
Algal whitening can devastate marine forests. However, algal whitening-causing coralline species may be used to prevent fouling by fleshy seaweed. Thus, biomimetic coralline algae may be applicable as an environmentally friendly material for anti-fouling coating treatment. To generate biomimetic coralline algae, it is necessary to include calcification inhibitors in the material to prevent the additional attachment and growth of living coralline species. Calcification is a critical process in plants because calcareous skeletons support and protect the soft parts of organisms, and secreted proteins play major roles in the photosynthetic assimilation of bicarbonate and nutrient acquisition (McConnaughey and Whelan, 1997). commercial calcification inhibitors were compared using TTC assays. Among them, ferric citrate or Fe(III) citrate showed the strongest inhibition against coralline cell viability. Ferric citrate as a hydroxyapatite inhibitor is also known to be a non-protein-bound iron transporter in plants (Solti et al., 2012) and animals (Baker et al., 1998). Part of the ferric ion undergoes reduction to ferrous iron mediated by ferric chelate reductases (Jeong et al., 2008). Ferrous citrate or Fe(II) citrate induces oxidative damage in mitochondria through lipid peroxidation and alterations in membrane proteins (Castilho et al., 1994). Under natural conditions, iron can be present as divalent or trivalent cations depending on the chemical environment, which makes it a good cofactor for oxidoreductase-type enzymes. Nevertheless, free ferrous ions are dangerous to living organisms as they can catalyze the Fenton reaction and produce reactive radicals (Winterbourn, 1995). Thus, at specific concentrations and in a localized area, ferric citrate may be used to prevent the additional settlement of coralline algae on biomimetic materials or to prevent the blooming of coralline algae.