An 8-week feeding trial was conducted to investigate the effects of dietary taurine supplementation on the growth performance of juvenile rock bream, Oplegnathus fasciatus. Triplicate groups of 25 fish averaging 2.72 ± 0.04 g (mean ± SD) were fed one of six experimental diets prepared by adding taurine at 0%, 0.25%, 0.5%, 1.0%, 1.5% and 3.0% (Control, Tau0.25, Tau0.5, Tau1.0, Tau1.5 and Tau3.0, respectively). At the end of the feeding trial, the weight gain, specific growth rate and protein efficiency ratio of fish fed the Tau0.5, Tau1.0 and Tau1.5 diets were significantly higher (P < 0.05) than those of fish fed the Control and Tau0.25 diets. The feed efficiency of fish fed the Tau0.5 diet was significantly higher than that of fish fed the Control, Tau0.25 and Tau3.0 diets. Fish fed the Tau1.0 diet had higher whole-body crude protein content than fish fed the Control diet, while the crude lipid content of fish fed the Tau1.5 and Tau3.0 diets was significantly lower than that of fish fed the Control and Tau0.25 diets. An ANOVA suggested that the optimum level of dietary taurine supplementation to improve growth and reduce the body lipid contents of juvenile rock bream, O. fasciatus, was 0.5%, while a broken line analysis of weight gain indicated a level of 0.62%.
Rock bream,
Taurine (2-aminoethanesulfonic acid) is a dietary supplement used to promote growth in aquaculture. It is the most abundant free amino acid in living organisms, and is involved in numerous important biological functions, including membrane stabilization, detoxification, anti-oxidation, modulation of the immune response, calcium transport, retina development, bile acid metabolism, osmotic regulation, and endocrine functions (Kuzmina et al., 2010; El-Sayed, 2013). Historically, taurine has not been considered an essential nutrient for fish; however, the taurine requirement in marine finfish was recently reported to be 2.6–72.0 mg taurine/g diet (Rhodes and Davis, 2011).
Dietary taurine supplementation to farmed finfish has been shown to reduce nutritional diseases, such as green liver, regulate hematocrit levels (Rhodes and Davis, 2011), and reduce body lipid content (Kim et al., 2008b; Espe et al., 2012), and is considered an essential nutrient for promotion of growth performance in several species (Park et al., 2002;Kim et al., 2003, 2005a, 2005b; Takagi et al., 2006, 2011; Gaylord et al., 2007; Lunger et al., 2007; Kim et al., 2008b; Qi et al., 2012), including rock bream (Lim et al., 2013).
The objective of this study was to investigate the effects of dietary taurine supplementation on the growth performance of juvenile rock bream,
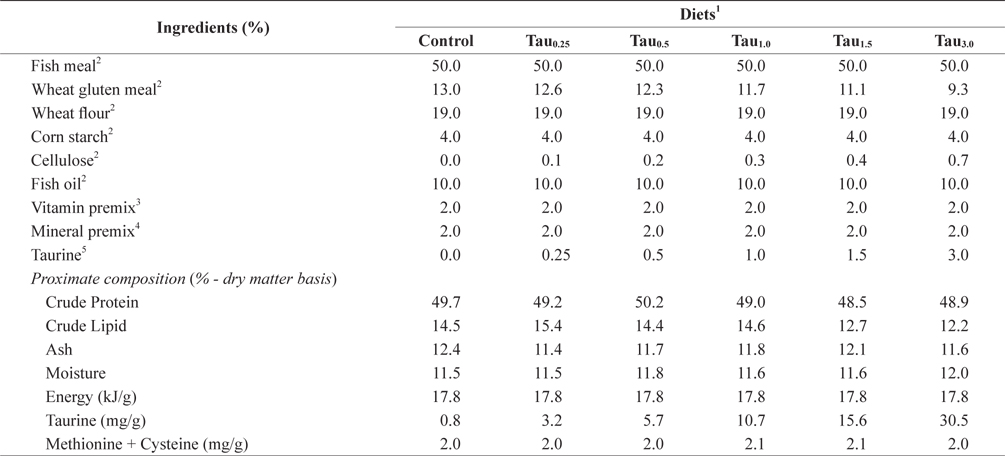
Six experimental diets using fish meal as the main protein source were prepared by adding taurine at 0%, 0.25%, 0.5%, 1.0%, 1.5% and 3.0% (designated as Control, Tau0.25, Tau0.5, Tau1.0, Tau1.5 and Tau3.0, respectively), as shown in Table 1. The experimental diets were formulated to be isonitrogenous (49% crude protein) and isocaloric (17 kJ/g). The energy levels of the diets were calculated based on 23.6, 17.2 and 39.5 kJ/g for protein, carbohydrate and lipids, respectively (NRC, 2011). The dietary cysteine and methionine concentrations were similar among the experimental diets. Dietary taurine (Korea Bio-gen, Cheonan, Korea) concentration (mg/g diet) increased proportionally with taurine supplementation level, and was 0.8 in Control, 3.2 in Tau0.25, 5.7 in Tau0.5, 10.7 in Tau1.0, 15.6 in Tau1.5 and 30.5 in Tau3.0. The experimental diets were prepared by thoroughly mixing the dry ingredients in an electric mixer, followed by addition of oil and distilled water. This mixture was formed into dough, and pellets were produced by passing the dough through a screw-type pelleting machine and air drying the formed pellets for 48 h. After drying, the pellets were ground, sieved into the required pellet size, packed and stored at −20°C until use.
[Table 1.] Formulation and proximate composition of experimental diets (% dry matter)

Formulation and proximate composition of experimental diets (% dry matter)
>
Experimental fish and feeding trial
Juvenile rock bream,
>
Sample collection and analysis
At the end of the feeding trial, the fish were starved for 24 h, then counted and weighed to calculate weight gain (WG), feed efficiency (FE), specific growth rate (SGR), protein efficiency ratio (PER) and survival. After the final weighing, three fish per tank were selected randomly and used for determination of the hepatosomatic index (HSI) and condition factor (CF). Random samples of 10 fish per tank at the beginning of the feeding trial and 10 fish per tank at the end of the trial were selected, minced and stored at −20°C until whole-body proximate composition and free amino acid (taurine, methionine and cysteine) analysis. The proximate compositions of the experimental diets and whole bodies of fish were analyzed according to standard methods (AOAC, 2006). Samples of the diets and fish were dried at 110°C for 24 h in a drying oven to determine their moisture content. Ash content was determined by incineration at 600°C in a muffle furnace. Crude protein content was determined using the Kjeldahl method (
All data were analyzed by one-way ANOVA. When a significant effect of the treatments was identified, a least significant difference (LSD) test was used to compare means. Treatment effects were considered significant at
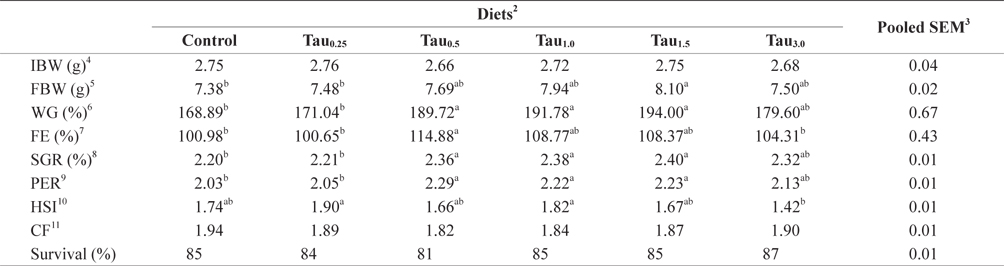
Percent weight gain (WG), feed efficiency (FE), specific growth rate (SGR), protein efficiency ratio (PER), hepatosomatic index (HSI), condition factor (CF) and survival rate data are summarized in Table 2. After the feeding trial, the WG, SGR and PER of fish fed the Tau0.5, Tau1.0 and Tau1.5 diets were significantly higher than those of fish fed the Control and Tau0.25 diets (
[Table 2.] Growth performance of juvenile rock bream fed the experimental diets for 8 weeks1
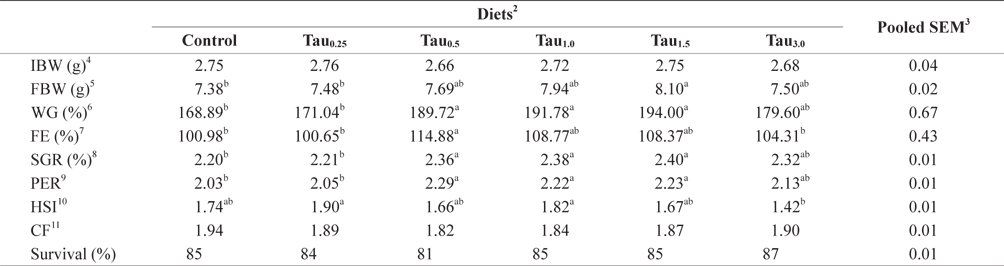
Growth performance of juvenile rock bream fed the experimental diets for 8 weeks1
Data on the proximate composition of juvenile rock bream fed the experimental diets are summarized in Table 3. The whole-body crude lipid content decreased steadily with increasing dietary taurine level. The whole-body lipid contents of fish fed the Tau1.5 and Tau3.0 diets were significantly lower than those of fish fed the Control and Tau0.25 diets; however, there were no significant differences in the whole-body lipid contents among fish fed the Tau0.5, Tau1.0, Tau1.5 and Tau3.0 diets, or among fish fed the Control, Tau0.25, Tau0.5 and Tau1.0 diets. Whole-body protein content trend to increase with the increasing of dietary taurine supplementation up to 1% and afterwards it was decreased. There were no significant differences in the moisture and ash contents among fish fed the experimental diets.
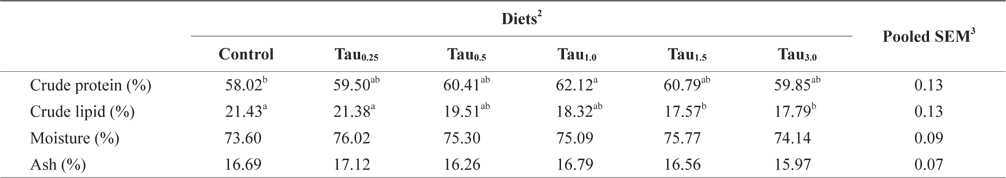
Whole-body proximate composition (% dry matter) of juvenile rock bream fed the experimental diets for 8 weeks1
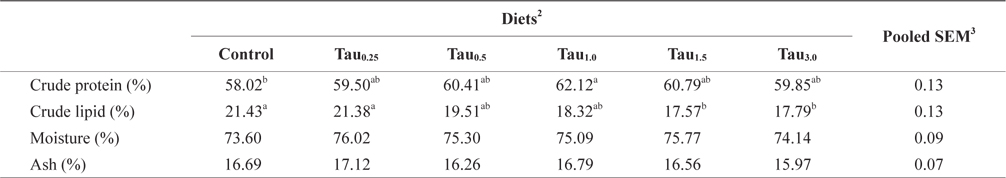
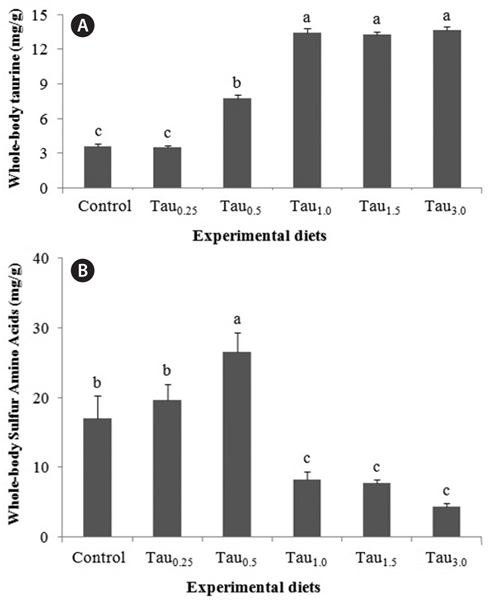
The taurine and sulfur amino acid (methionine plus cysteine) whole-body concentrations in fish fed the experimental diets are presented in Fig. 3A and 3B, respectively. The whole-body taurine content of fish fed the experimental diets increased with increasing dietary taurine until reaching a pla teau for the Tau1.0 diet. Fish fed the Tau1.0, Tau1.5 and Tau3.0 diets had a significantly higher whole-body taurine content than fish fed the other experimental diets. Conversely, the whole-body sulfur amino acid content increased with increasing dietary taurine up to Tau0.5, and then declined steadily. Fish fed the Control, Tau0.25 and Tau0.5 diets had significantly higher whole-body sulfur amino acid contents than fish fed the Tau1.0, Tau1.5 and Tau3.0 diets.
Discussion
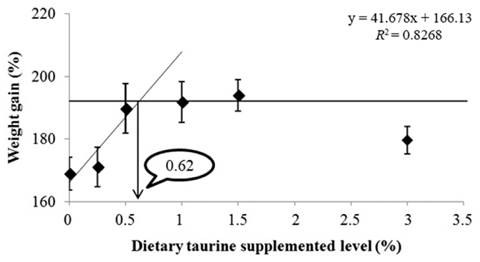
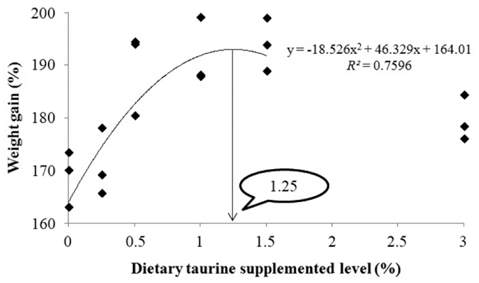
Our results suggested a positive effect of dietary taurine on the growth performance of juvenile rock bream. ANOVA of the tested growth parameters indicated no benefits of supplementation with more than 0.5% taurine; fish fed diets with higher levels of taurine did not display any further improvement in growth performance. However, broken line regression analysis based on WG indicated an optimum dietary taurine supplementation level of 0.62%, which corresponds to a requirement of 7.0 mg taurine/g diet. Moreover, second-degree regression analysis of WG indicated that the maximum dietary taurine supplementation level was 1.25%, which indicates that juvenile rock bream can effectively utilize up to 13.3 mg taurine/g diet. The growth performance results indicated that excessive taurine supplementation (Tau1.0 and Tau1.5) did not reduce growth compared with the optimum level of taurine supplementation (Tau0.5). However, supplementation with the highest dietary taurine level (Tau3.0) resulted in a significant decrease in growth, suggesting that excessive taurine supplementation retards growth by reducing feed intake, as has been reported for olive flounder
Dietary taurine supplementation has not only shown positive effects on growth performance but has also been reported to be essential in several species. Park et al. (2002) and Kim et al. (2005a, 2005b) reported that the dietary taurine level for the optimum growth of olive flounder ranges from 14–16 mg taurine/g diet. Also, flounder fed diets supplemented with 10 mg taurine/g diet exhibited superior growth, not only relative to a basal diet, but also to those diets supplemented with gamma-aminobutyric acid (GABA) and β-alanine (Kim et al., 2003). Qi et al. (2012) suggested 10 mg taurine/g diet as the dietary taurine requirement for normal growth and feed efficiency in turbot. Similar to our results, Qi et al. (2012) reported that with increasing dietary taurine intake, the growth performance increased and then plateaued, with no beneficial effects of taurine supplementation greater than the required level. Additionally, the taurine requirement for growth and feed efficiency enhancement of red sea bream,
Taurine supplementation has been reported to be essential for rock bream (Lim et al., 2013). A fish-meal-based diet was used as a control, and diets supplemented with 0.2%, 0.4%, 0.8%, 1.2% or 1.6% taurine were fed to triplicate groups of juvenile rock bream (initial body weight of 13.5 g) to apparent satiation for 8 weeks. Similar to our results, the fish readily accepted all of the experimental diets and maintained normal behavior during the feeding trial. The growth and feed utilization were significantly improved by taurine supplementation up to 0.8%, but plateaued thereafter. The optimum dietary taurine level was determined by a broken line regression analysis based on weight gain to be 0.88% (8.8 mg taurine/g diet). However, their results suggested no significant differences in WG, SGR, FE and PER between fish fed 0.8% and 0.4% taurine-supplemented diets.
In our study, the taurine content in whole-body increased with increasing dietary taurine until fish fed taurine at 1% and then reached a plateau. Similarly, Park et al. (2002), Kim et al. (2003, 2005a, 2005b, 2008b), Matsunari et al. (2008) and Qi et al. (2012) reported a positive linear relationship between whole-body taurine accumulation and the dietary taurine level. The major pathway for taurine synthesis from methionine in mammals is believed to involve the transformation of methionine to cystathionine by cystathionine synthetase, transformation of cystathionine to cysteine by cystathionase, oxidation of cysteine to cysteinesulfinate, decarboxylation of cysteinesulfinate to hypotaurine, and further oxidation of hypotaurine to taurine (Worden and Stipanuk, 1985). Therefore, the low whole-body taurine levels of fish fed the Control and Tau0.25 diets suggest a deficiency in taurine biosynthesis in juvenile rock bream. The opposite relationship was found for sulfur amino acid whole-body content; methionine and cysteine contents decreased with increasing taurine supplementation. A reduction in whole-body cystathionine content with increasing dietary taurine level has been reported for olive flounder (Park et al., 2002). Similarly, Kim et al. (2003, 2005a) suggested that cystathionine accumulates in the tissues of flounder fed a diet without taurine supplementation and that the accumulated cystathionine may be used for taurine biosynthesis. The results of our previous study of the taurine biosynthesis pathway (Worden and Stipanuk, 1985), and research into rock bream (Lim et al., 2013), suggest a taurine insufficiency in the Control and low taurine (Tau0.25) diets. Indeed, the accumulation of sulfur amino acids in the whole-body of rock bream fed a taurine-insufficient diet suggests that juvenile rock bream accumulate sulfur amino acids to facilitate posterior taurine biosynthesis.
In our study, while the whole-body crude protein content of fish fed the experimental diets was affected by taurine only slightly, the crude lipid content displayed a linear decrease with increasing dietary taurine level. Similar results were reported in flounder (Kim et al., 2008b), Atlantic salmon (Espe et al., 2012), turbot (Qi et al., 2012) and red sea bream (Matsunari et al., 2008). According to Espe et al. (2012), the lower crude lipid whole-body content indicates that the addition of a low concentration of taurine to high-plant-protein diets influences lipid metabolism and storage, concomitantly affecting general metabolism. In particular, taurine plays a role in fat digestion by conjugating bile acids, such as cholic acid or chenodeoxycholic acid, in the liver. In lipid metabolism, taurine is involved in the degradation of cholesterol metabolites and participates in micelle formation, thus contributing to fat absorption in the small intestine (Yokogoshi et al., 1999).
In summary, the optimum dietary taurine level to improve the growth of juvenile rock bream,