Candida species, commonly isolated from clinical material, are a common cause of fungal infections in humans. The most pathogenic Candida species are C. albicans, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis. Among these, C. albicans is generally responsible for 90–100% of mucosal infections and for 50–70% of candidemia episodes. Since Wilkinson first described the association between fungus and vaginal discharge (candidiasis) in 1849, many antifungals such as compounds of polynes and azoles have been used for treatment. Among the drugs used in treating Candida infections, fluconazole (an orally active triazole agent) is well established as a first-line management option for both localized and systemic infections. However, antifungal use during the past few years has aided in the development of strains resistant to many antifungal antibiotics. Side effects associated with fluconazole include nausea, vomiting, diarrhea, and liver enzyme elevation.
As alternatives with fewer side effects, medicinal plants and marine organisms have been investigated for candidiasis treatment. Eisenia bicyclis is a common perennial in the group Phaeophyceae (brown alga), and is generally distributed in the region of Ulleung island in the East Sea of Korea. It has been added to appetizers, casseroles, muffins, pilafs, and soups (Maegawa, 1990; Yoon et al., 2011). The antioxidant activities of E. bicyclis phlorotannins, such as eckol (a trimer), phlorofucofuroeckol A (a pentamer), dieckol, and 8,8’-bieckol (hexamers), have been previously described (Okada et al., 2004). Additionally, several medicinal functions of E. bicyclis have been reported, including antitumor (Ermakova et al. 2013), anti-Alzheimer’s disease (Ahn et al., 2012), antiatherosclerosis (Kang et al., 2006), anti-inflammatory (Jung et al., 2013), anticoagulant (Jeong et al., 2009), antiallergic disease, and anticancer activities (Shibata et al., 2003; Yoon et al., 2013). Phlorotannins have also been known to show potent antimicrobial activity against several microorganisms (Eom et al., 2013). However, very little research has been done on the antifungal activity of E. bicyclis against Candida species. Here, we demonstrated that E. bicyclis methanol (MeOH) extract and its solvent-soluble form have high antifungal effects against Candida species, and may act as alternative and therapeutic agents for candidiasis.
In late September 2010, E. bicyclis was purchased from Ulleung Trading Co. (Ulleung-Gun, Korea). A voucher specimen was deposited in the author’s laboratory. Dried E. bicyclis was finely ground and powdered with a food mixer (HMF- 1000A; Hanil Electronics, Seoul, Korea). The dried powder was vacuum-packed and kept at –20°C until use. The dried E. bicyclis powder (1.0 kg) was extracted with MeOH (10 L × 3) at 70°C for 3 h (3 times), and the solvent was evaporated in vacuo with a rotary evaporator (N-1001S-W; Eyela, Tokyo, Japan). The crude MeOH extract of E. bicyclis was suspended in 10% MeOH (1.0 L) and partitioned in turn with n-hexane (Hexane), dichloromethane (DCM), ethyl acetate (EtOAc), and n-butanol (BuOH) in sequence. The concentration of each extract was adjusted to 500 mg/mL by dissolving in dimethyl sulfoxide (DMSO) under sterile conditions and stored at –70°C until used.
In this study, we used the yeast strain C. albicans from the Korean Collection for Type Cultures (KCTC; Daejeon, Korea). Candida albicans and C. glabrata clinical isolates from a variety of body sites were provided by the Gyeongsang National University Hospital (Jinju, Korea), a member of the National Biobank of Korea. The isolates were maintained at 4°C on Sabouraud Dextrose Agar (Difco, Franklin Lakes, NJ, USA) plates and subcultured for 24 h at 37°C in Sabouraud Dextrose Broth (SDB; Difco) before each experiment to ensure viability. The disk diffusion assay was prepared in Mueller–Hinton agar (MHA; Difco) supplemented with 2% glucose and 0.5 μg/mL of methylene blue, and the broth dilution method was carried out in RPMI-1640 supplemented with 2% glucose (Difco) according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (2004; formerly NCCLS).
Antifungal efficacy was evaluated via disk diffusion assays, as described by the CLSI (2004). In brief, Candida species were cultured in SDB at 37°C until cells reached an OD600 nm of 0.5. One hundred microliters of fungal culture containing approximately 104–105 CFU/mL was spread on MHA plates. A paper disc (8 mm in diameter) containing 50 mg of each extract was placed on the plates. After incubation for 24 h at 37°C, the diameter of the inhibition zone was measured on fungal culture plates. The experiment was carried out three times and mean values are presented.
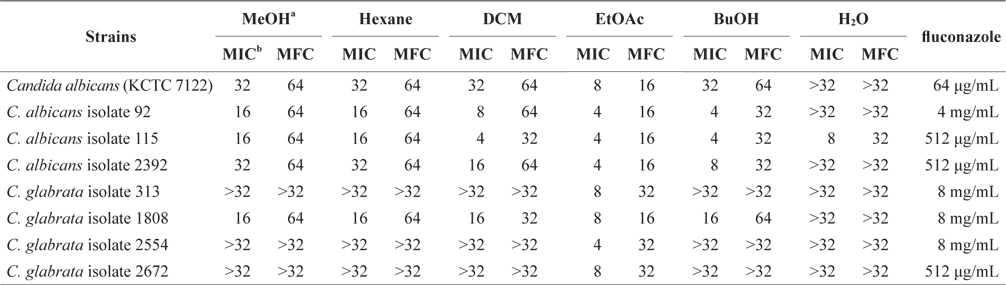
The concentrations of E. bicyclis MeOH extract and its solvent-soluble extracts were both 500 mg/mL. To determine the minimum inhibitory concentration (MIC) values of the MeOH extract and its solvent-soluble extracts, each extract was diluted with RPMI-1640 supplemented with 2% glucose to obtain a stock solution of 1, 2, and 4 mg/mL. The MICs were at the lowest concentrations of MeOH and solvent-soluble extracts to inhibit the visible growth of microorganisms after aerobic incubation for 48 h using RPMI-1640 supplemented with 2% glucose, modified from the guidelines of CLSI document M27-A3 (CLSI, 2008). MICs of the solventsoluble extracts were determined by the twofold serial dilution method in 96-well flat-bottomed microtitration plates at a final concentration of 5 × 105 CFU/mL. The microtitration plates were read visually and the MIC was recorded as that of the extracts that exhibited no turbidity. For minimum fungicidal concentration (MBC) testing, an aliquot of inoculum was taken with a MIC test well that did not show turbidity, and was poured onto SDA agar (Difco) plates with Candida species. The agar plates were incubated at 37°C until growth was detected in the growth control plates. The MFC value was defined as the lowest concentration required to kill 99.99% or more of the initial inoculum. The MIC and MFC experiments were repeated thrice.
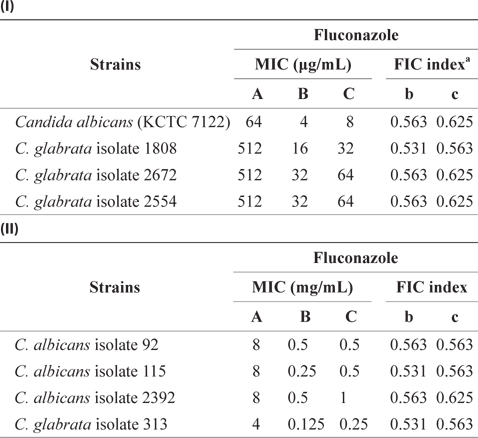
The interaction between the EtOAc-soluble extract from E. bicyclis and antibiotic fluconazole (Sigma Chemical Co., St. Louis, MO, USA) against Candida species was tested by the checkerboard method (Perea et al., 2002; Weig and Muller, 2001). The synergistic effect was evaluated as a fractional inhibitory concentration (FIC) index. With the checkerboard test, the FIC was calculated as the MIC values of an antibiotic or EtOAc-soluble extract in combination and divided by the MIC of the antibiotic or EtOAc-soluble extract alone. The FIC was then summed to derive the FIC index, which indicated synergy. Index values were determined using the following formulas: FICA = MICA in combination/MICA, FICB = MICB in combination/MICB, FIC Index = FICA + FICB
where A and B are the MICs of drug A and compound B in the combination, MICA and MICB are the MICs of drug A and compound B alone, and FICA and FICB are the FICs of drug A and compound B.
The interaction was defined as synergistic if the FIC index was <1, additive if the FIC index was 1.0, subadditive if the FIC index was between 1.0 and 2.0, indifferent if the FIC index was 2, and antagonistic if the FIC index was >2. Synergy was further subclassified as marked (FIC index ≤ 0.50) or weak (FIC index between 0.50 and 1.0).
In all cases, analyses were performed in triplicate and data were averaged. We calculated standard deviation for all measurements. We used Student’s t-test (α = 0.05) in SPSS 12.0 (SPSS Inc., Chicago, IL, USA) to test differences between MIC values for each individual microorganism.
Fluconazole has been widely used for the treatment of candidiasis. However, it can cause numerous side effects, including nausea, vomiting, and headache, as well as liver damage and altered estrogen (Lilly, 2012). In addition, increasing reports of fluconazole-resistant C. albicans strains have been published (Pfaller, 2012). Therefore, a need exists to develop new medicines or alternative therapies for candidiasis.
In an effort to decrease antibiotic use and discover an alternative therapeutic agent for treating Candida infections, we tested the MeOH extract and its soluble extracts from E. bicyclis, a brown alga. The relative susceptibility of Candida species to potential antimicrobial agents was measured by a clear zone of growth inhibition around the disc.
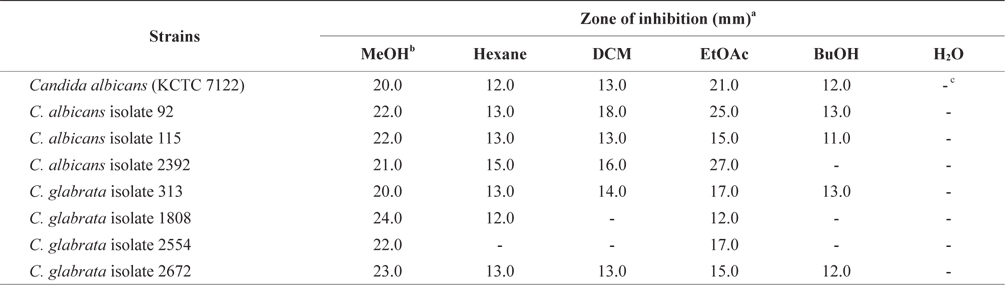
The antifungal activity of the MeOH extract and its solventsoluble extracts are presented in Table 1. The MeOH extract of E. bicyclis exhibited antifungal activity against Candida species, suggesting that the extract contained antifungal substances. For C. albicans, the clear zones of treatment with the EtOAc-soluble extract had a diameter of 21.0 mm and a concentration of 50 mg per disc. For C. albicans isolates, the antifungal activity of the EtOAc-soluble extract was 50 mg per disc (diameter of inhibition: 15–25 mm). For C. glabrata isolates, the antifungal activity of the EtOAc-soluble extract was also 50 mg per disc (diameter of inhibition: 12–17 mm). However, the water-soluble form of the MeOH extract did not exhibit antifungal properties against all Candida species. In Table 1, we show different patterns of antifungal activities for the same species. These differences may be due to the presence of additional mechanisms of resistance. According to Rodloff et al. (2011), different Candida species may vary in their susceptibility to antifungals.
Khaled et al. (2012) showed that the EtOAc extract of the brown alga Padina pavonica showed significant antifungal activity against C. glabrata (diameter of inhibition: 16 mm) and C. krusei (diameter of inhibition: 14 mm). The sterile disks were impregnated with different extracts and dried (25 μL/ disc). Thus, E. bicyclis displays a similar antifungal activity against Candida species as other brown seaweeds.
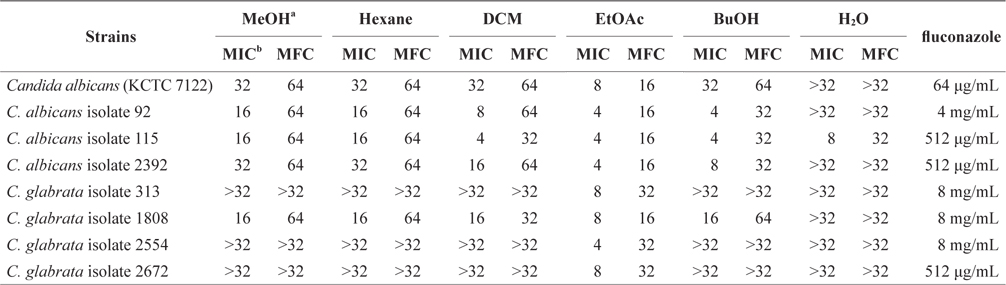
To quantitatively evaluate antifungal activity, we investigated the MIC and MFC values of the MeOH extract and its solvent-soluble extracts. The MIC values of five solventsoluble extracts against Candida species varied depending on the polarity of the solvent. Among five solvent-soluble extracts, the EtOAc-soluble extract showed the lowest MIC values against Candida species. The EtOAc-soluble extract was able to completely inhibit the growth of Candida species at concentrations of 4 and 8 mg/mL. The antifungal activity of the EtOAc-soluble extract against Candida species was higher than that of other soluble extracts. Lopes et al. (2013) reported the antifungal activity of the purified phlorotannins extracts from the brown seaweed Cystoseira nodicaulis against C. albicans. The MIC values of purified phlorotannin extracts of C. nodicaulis were 15.6 mg/mL against C. albicans (American Type Culture Collection strain 10231) and 62.5 mg/mL against C. albicans isolates. In this study, the EtOAc-soluble extract of E. bicyclis exhibited increased antifungal activity against C. albicans in comparison to that of C. nodicaulis. The MFC values of the EtOAc-soluble extract against Candida species were 16–32 mg/mL (Table 2). In contrast, the watersoluble extract did not exhibit antifungal activity against Candida species. According to Eom et al. (2013), marine-derived polyphenols (phlorotannins) are believed to be the active components of E. bicyclis. These are the predominant EtOAcsoluble compounds in brown algae (Choi et al., 2010). Among EtOAc-soluble compounds, polyphenol polymers (eckol, phlorofucofuroeckol A, dieckol, and 8,8′-bieckol) exhibited potent antibacterial activities (Isnansetyo and Kamei, 2009; Nagayama et al., 2002). Differing patterns of antifungal activity were also observed in MIC and MFC values within species. Although the underlying mechanisms of these solvent-soluble extracts were not completely elucidated, evidence exists indicating effects on ergosterol and chitin composition in filamentous fungi, and ergosterol and respiration in yeast (Lopes et al., 2013). Therefore, further research on E. bicyclis methanol extract and its solvent-soluble extracts against Candida species may provide clues regarding susceptibility to candidiasis.
In addition, we found Candida isolates to be highly resistant to the antibiotic fluconazole in this study. Among Candida isolates, C. glabrata isolates with high-level resistance to fluconazole were detected, with MIC values of 8 mg/mL. Thus, further studies are needed to elucidate the main components of E. bicyclis against Candida species.
Natural materials such as plant- or marine-derived compounds in combination with traditional medicines can be used as effective approaches for restoring antibiotic activity in treatments against drug-resistant bacteria (Eom et al., 2013). Sharma et al. (2010) reported that pure polyphenol curcumin I from Curcuma longa exhibited a marked synergy with amphotericin B antibiotics against C. albicans. Riccardin C, isolated from the Chinese liverwort Plagiochasm intermedium L., also showed synergistic or additive activity when combined with fluconazole against fluconazole-resistant C. albicans strains (Xie et al., 2010). Based on these reports, the synergistic effects of marine-derived polyphenol on Candida species were assessed in combination with commercial antibiotics to treat candidiasis.
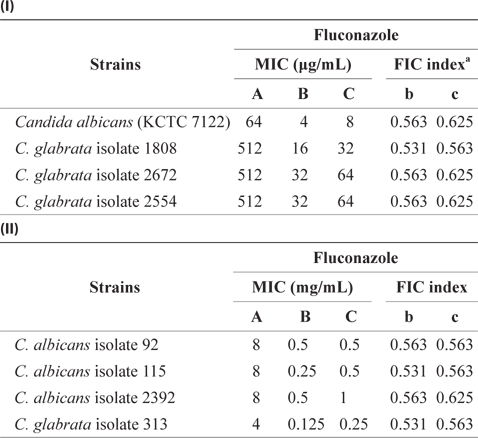
Our results in Table 2 reveal that Candida isolates are resistant to fluconazole. Due to their resistance to commonly used antibiotics, a need exists for more effective antifungal agents. Thus, the FIC test for the combination of EtOAc-soluble extract and antibiotics was assessed using the checkerboard test. According to the results displayed in Table 3, the MIC values of fluconazole against C. albicans KCTC 7122 decreased from 64 to 4 μg/mL when fluconazole administered in combination with 4 mg/mL of the EtOAc-soluble extract. The MIC values of fluconazole against Candida isolates were also greatly diminished when administered in combination with the EtOAcsoluble extract. The FIC indices of antibiotics were between 0.531 and 0.536 in combination with the concentration of the EtOAc-soluble extract (1.0–4.0 mg/mL) against Candida isolates, thereby indicating the marked synergistic inhibitory effect of the EtOAc-soluble extract and fluconazole against the growth of Candida species. Thus, the results of the checkerboard assay revealed a restoration of antifungal activity against an antibiotic-resistant Candida species when used in combination with the EtOAc-soluble extract from E. bicyclis.
In conclusion, we evaluated the antifungal activity of the edible marine brown alga E. bicyclis against Candida species. Since the EtOAc-soluble extract showed the strongest antifungal activity against Candida species among five solventsoluble extracts, the antifungal activity of E. bicyclis extracts may correlate with phlorotannins or marine-derived polyphenolic contents. Although the EtOAc-soluble extract from E. bicyclis has been shown to have less antifungal activity compared with commercial antibiotics, E. bicyclis can be utilized as an effective, safe, and natural source of antifungal agents. The EtOAc-soluble extract in combination with antibiotics is expected to have an additive therapeutic effect for relieving symptoms against Candida species. The results of the present investigation will contribute to the development of an alternative phytotherapeutic agent against antibiotic-resistant Candida species.