The present study was conducted to elucidate effects of heat, salt and hydrocolloid treatments on flying fish Cypselurus agoo roe analogs prepared using calcium alginate gel. The changes in size, sphericity and rupture strength of the analogs as affected by treatments of heat, sodium chloride and hydrocolloids were investigated. The size (mm), sphericity (%), and rupture strength (kPa) of the analogs were 2.2 ± 0.1, 98.2 ± 0.2, and 74.7 ± 1.7, respectively. When the analogs were heated at 95℃ in water, the size was slightly decreased. The rupture strength by curing with 2% sodium chloride was slightly increased. Sphericity didn’t show significant differences by sodium chloride and heat treatment. The rupture strength of the analogs was slightly decreased by heat treatment, whereas remarkably decreased by curing with sodium chloride. In order to prevent a remarkable decrease in rupture strength of the analogs by curing with sodium chloride, the analogs were treated with hydrocolloids such as xanthan gum, gum guar, glucomannan, pectin and gelatin. The hydrocolloids treated analogs showed an increment in size and no significant changes in sphericity. On the other hand, the rupture strength of the hydrocolloids treated analogs exhibited remarkable increase than that of untreated ones.
Alginate is mainly extracted from brown algae and one of the most widely used biopolymer for hydrogels. It is consisted of linear binary copolymers of 1→4 linked β-D-mannuronic acid (M) and α-L-guluronic acid (G) residues (Voo et al., 2011). Alginate is an excellent biopolymer possessing properties such as good biocompatibility, good biodegradability, low immunogenicity, non-toxicity and environmental friendly (Rowley et al., 1999; Becker et al., 2001; Martins et al., 2007; Yang et al., 2007).
Alginate forms a thermally stable and biocompatible hydrogels in the presence of di- or tri-cations (Chan et al., 2011). The gel formation occurs due to an ionic interaction between G residues from two or more alginate chains and multivalent cations (Augst et al., 2006). The properties of alginate gels are strongly dependent on the ratio of uronic monomers, sequence, cation concentration in the reaction solution, and stabilization time (Smidsrød, 1974; Draget et al., 1994; Velings and Mestdagh, 1995; Ouwerx et al., 1998). Such properties increase water holding capacity in organisms naturally, maintains cells from cultured parent. Alginate also possesses the characteristics of both liquid and gel as it is composed of unique biopolymers compared to other polysaccharides (Draget et al., 1997).
Hydrogel beads are applied in many industrial fields such as in the field of biomedical, bioprocess, pharmaceutical, and food industry (Chan et al., 2011). Especially, the application in the biotechnology as well as bio-medical areas is suitable for in vitro culture by regeneration function as well as for the applications of biological tissue engineering (Duff, 1985; Clayton et al., 1993; Colton, 1995; Paige et al., 1996). In addition, it is applied as an immobilization matrix for the encapsulation of biomaterials such as pancreatic islets, and probiotic bacteria and controlled delivery applications (Draget et al., 1997; Brekken et al., 2004). Alginate gel is also capable to be utilized in the encapsulation of highly viscous high-fat food (Blandino et al., 1999).
In the food industry, encapsulation using alginates is most often carried out by producing alginate gel via the extrusion-dripping method. An alginate solution is extruded through a capillary and allowed to break away from the nozzle in droplet form under the influence of gravitational force or external force (Lee et al., 2013). Due to electrostatic interactions with calcium ions, alginate gelation is a phenomenon which creates three-dimensional “egg-box” structure owing to the cross-linking of sodium ions in guluronic acid when calcium ions are added into alginate solution (Clark and Ross-Murphy, 1987; Vandenberg and Bell, 2001; Rousseau et al., 2004). The production of fish roe analogs using alginate gel was often influenced some trial-and-error works on the liquid formulation and experimental set-up (Ji et al., 2007; Woo et al., 2007). So, processing of the analogs is required to improve their physical properties such as size, sphericity, rupture strength etc. according to processing conditions.
The present study was conducted to elucidate effects of treatments of heat, salt and hydrocolloid on flying fish roe analogs prepared using calcium alginate gel. The changes in size, sphericity and rupture strength of the analogs as affected by treatments of heat, sodium chloride and hydrocolloids were investigated.
Sodium alginate (Junsei Chemical Co., Ltd., Tokyo, Japan) and anhydrous calcium chloride (Junsei Chemical Co., Ltd.) were used for alginate gel. Gum guar, xanthan gum, and glucomannan were provided by MSC Co. (Yangsan, Korea). Gelatin and pectin were purchased from Sigma-Aldrich (St. Louis, MO, USA). All chemicals and reagents used in this study were analytical grade.
>
Preparation of flying fish roe analogs
Sodium alginate (2.6%, w/v) was dropped into 1.5% (w/v) calcium chloride at a flow rate of 0.06 mL/s using SMP-23 Cassette tube peristaltic pump (Eyela, Tokyo, Japan) through the 26G (1/2 inch) nozzle that was connected to a silicon tube. The distance between the nozzle and the surface of the calcium chloride solution was 8 cm. The stirring speed of the reactor in calcium chloride solution was set at 300 rpm. The sodium alginate was dropped every 2 min and stabilized for 31 min in the solution. The analogs were separated from the solution using a strainer and then washed by distilled water and stored at an ambient temperature.
>
Salt and heat treatment of the analogs
Salt treatment was performed by curing the analogs in 2.0% (w/v) sodium chloride solution and then physical properties including size, sphericity, and rupture strength of the analogs were determined by Image-Pro Plus (Media Cybernetics, Inc., Bethesda, MD, USA), Bx-50 optical microscope (Olympus, Tokyo, Japan), and CR-100D rheometer (Sun Scientific Co., Ltd., Tokyo, Japan). Heat treatment was carried out by boiling the analogs at 95°C and then the physical properties were determined.
>
Hydrocolloid treatment of the analogs
Hydrocolloids such as gum guar, xanthan gum, pectin, glucomannan and gelatin were used to treat the analogs. The analogs were immersed in 1% (w/v) hydrocolloids maintained at 90°C for about 1 min. The hydrocolloids treated analogs were heated at 90°C in 2.0% (w/v) sodium chloride solution. The physical properties including size, sphericity, and rupture strength were measured by Image-Pro Plus (Media Cybernetics, Inc.), Bx-50 optical microscope (Olympus), CR-100D rheometer (Sun Scientific Co., Ltd.).
The size of the analogs was measured by an Image-Pro Plus image analyzer (Media Cybernetics, Inc., Bethesda, MD, USA) coupled to a Bx-50 optical microscope (Olympus, Tokyo, Japan). The image analysis of the analogs was produced at ×40 magnification. For the size of the analogs, the analogs (5 per each) were randomly selected and then the average of their maximum diameter and minimum diameter was measured.
Sphericity was expressed as the percent ratio of minimum diameter to maximum diameter that was obtained from the diameter measurement of the analogs,
>
Measurement of rupture strength
Rupture strength was measured using a CR-100D rheometer (Sun Scientific Co., Ltd., Tokyo, Japan) with the following conditions: round-disk stainless steel plunger and 5 mm diameter. Five samples were used per experiment.
All the results were subjected to analysis of variance, ANOVA (
>
Changes in physical properties of the analogs by heat and salt treatment
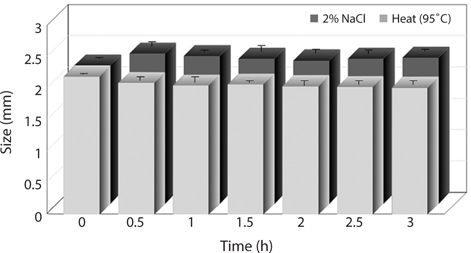
The changes in size of the analogs by heat and salt treatment as affected by different treatment time were shown in Fig. 1. Generally, heat and salt treatment is used to enhance storage stability and taste of products. The size of the analogs slightly decreased by heat treatment at 95°C for 30 min compared to intact analogs without heat treatment. Whereas there was no more decrease in size observed when the heat treatment was continued more than 1 h. Such decrease in size of the analogs is considered to be due to dehydration resulting from denaturation of hydrocolloid during the heat treatment. On the other hand, the analogs treated with 2% NaCl for 30 min was slightly increased in size. This is caused by the characteristic of alginate gel which absorbs sodium as well as moisture existing in the sodium solution at the same time (Bang et al., 2002). Moreover, when the analogs were treated with 2% NaCl solution more than 30 min, the significant difference was not exhibited in the size of the analogs.
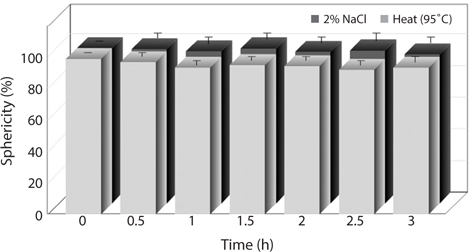
The changes in sphericity of the analogs by heat and salt treatment as affected by different treatment time were shown in Fig. 2. The sphericity (%) of the intact analogs produced was 98.2 ± 0.2, indicating that it was very close to a sphere. The sphericity of the analogs was slightly decreased by heat treatment. In addition, the sphericity of the analogs based on the time period of heat treatment was high (92% to 98%). On the other hand, the analogs treated with 2% NaCl showed no the significant differences in the sphericity of the analogs. Even though there was difference in sphericity depending on the time period of heat and salt treatment, the difference was not remarkable.
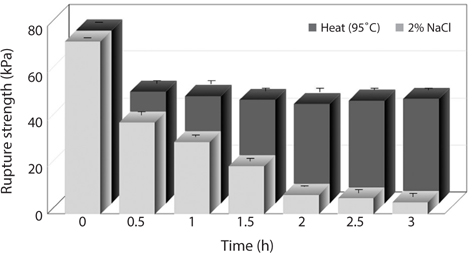
The changes in rupture strength of the analogs by heat and salt treatment as affected by different treatment time were shown in Fig. 3. The rupture strength of the intact analogs prepared was 74.7 ± 1.7 kPa. When the analogs were heated at 95°C, the rupture strength was reduced. The rupture strength of the analogs was decreased until 47.3 kPa by heat treatment for 30 min but was not decreased when the heat treatment was continued more than 1 h. On the other hand, the rupture strength of the analogs treated by 2% NaCl noticeably decreased with the time elapsed. Generally, alginate forms a thermally stable and biocompatible hydrogels in the presence of calcium ion (Chan et al., 2011). The gel formation occurs due to an ionic interaction between G residues from two or more alginate chains and multivalent cations (Augst et al., 2006). Due to electrostatic interactions with calcium ions, alginate gelation is a phenomenon which creates three-dimensional “egg-box” structure owing to the cross-linking of sodium ions in guluronic acid when calcium ions are added into alginate solution (Clark and Ross- Murphy, 1987; Vandenberg and Bell, 2001; Rousseau et al., 2004). By the treatment of NaCl, the chloride ion induces the liberation of calcium ion bound to G residues of calcium-alginate gel. As a result, it is considered that the rupture strength of the analogs by treatment of salt remarkably decreased with the time elapsed. In order to keep rupture strength of the analogs, the prevention of liberation of calcium ion from the analogs is needed.
>
Changes in physical properties of the analogs treated with hydrocolloids by salt
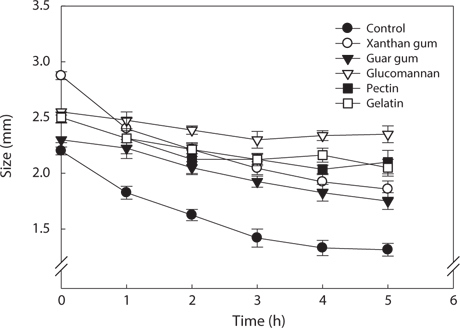
Generally, hydrocolloids such as xanthan gum, gum guar, glucomannan, pectin, and gelatin are much used as thickening and coating agents in food industry. In order to prevent the decrease in rupture strength of the analogs by salt treatment, the analogs were treated with hydrocolloids. Changes in size, sphericity and rupture strength of the analogs by treatment with hydrocolloids were investigated. The changes in size of the hydrocolloid-treated analogs by salt as affected by different treatment time were shown in Fig. 4. Intact analogs were remarkably decreased in size than the hydrocolloid-treated analogs by salt. The hydrocolloid-treated analogs were slightly increased in size and showed no the significant differences in size with the time elapsed. The size of the hydrocolloid-treated analogs was insignificantly affected by kinds of hydrocolloids.
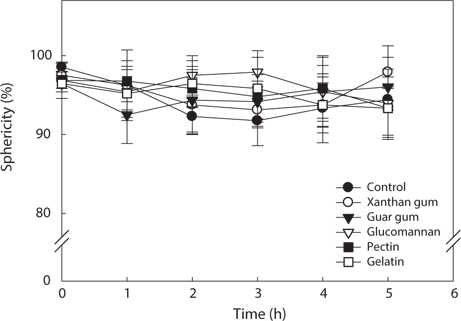
The changes in sphericity of the hydrocolloid-treated analogs by salt as affected by different treatment time were shown in Fig. 5. Noticeable reduction in sphericity of the hydrocolloid-treated analogs was not observed. The sphericity was greater than 90% in most of the cases and significant differences were not exhibited between the kinds of hydrocolloids.
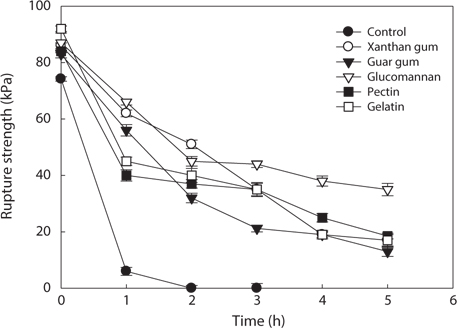
The rupture strength of the hydrocolloid-treated analogs by salt as affected by different treatment time was shown in Fig. 6. Extremely weak rupture strength was observed when salt was treated in the intact analogs without hydrocolloids treatment. After 1.5 h, it was incapable to measure rupture strength of the intact analogs because the gel was too weak to hold the shape. The rupture strength of hydrocolloid-treated analogs by salt treatment was decreased with time elapsed. The extent of the decrease was lower than that of the intact analogs. The decrease in rupture strength of analogs by salt was inhibited by treatment of hydrocolloids. Among hydrocolloids, glucomannan showed better rupture strength. It is considered that when the analogs were cured with salt, the treatment of hydrocolloids provides better rupture strength than that of the intact analogs.