Elucidation of biological diversity within ecosystems is fundamental to our understanding of ecosystem structure and function. Biodiversity is mainly determined by the composition of resident species within the system. Species that are beyond the detection limits of conventional monitoring methods frequently cause under-estimation of diversity. Therefore, developing more effective monitoring processes is an urgent issue in biodiversity research. This is particularly important for ecosystems with highly sustained habitat heterogeneities and unusually large biodiversities.
Freshwater macrophytes have deterministic effects on the habitat structures of water, and result in the construction of heterogeneous mosaics on different scales (O’Hare et al. 2006, Smokorowski and Pratt 2007). Habitat heterogeneity may create additional niches and diverse ways of exploiting environmental resources for resident animals (Bazzaz 1975) by providing them with refuges from predators, suitable spawning, and foraging substrates, thereby resulting in a higher number of animal individuals (Vieira et al. 2007). Microcrustaceans depend largely on aquatic macrophytes and can effectively utilize macrophyte habitats (Burks et al. 2002, Kuczyńska-Kippen and Nagengast 2006). Habitat heterogeneity leads to differences in the species composition and spatial distribution of microcrustacean communities (Meerhoff et al. 2006, Thomaz et al. 2008). Given the importance of microcrustaceans in aquatic food webs (i.e., in the transfer of energy and materials), the precise determination of the species composition of microcrustacean communities is central to limnological research, but it is frequently hindered by excessive macrophyte development.
Planktivorous fish spend a large proportion of their time in foraging activity and therefore encounter a high number of microcrustacean species within an ecosystem. In general, the presence of macrophytes is believed to decrease prey capture by fish (Meerhoff et al. 2006, 2007); however, some fish species have developed specialized abilities to forage in vegetated habitats (Jacobsen et al. 1997). For example, the bluegill sunfish (
In the present study, we determined the microcrustacean species composition in an ecosystem with excessive macrophyte development, by using fish gut analysis. We hypothesized that the number of microcrustacean species would be higher in fish guts than in the field. To test our hypothesis, we compared the microcrustacean species compositions in the field with those in the guts of bluegill sunfish.
South Korea is located in East Asia and has a temperate climate. Four distinct seasons lead to dynamic succession among biological communities inhabiting the country’s freshwater ecosystems. In the present study, we monitored Jangcheok Lake, which is located in the southeastern part of South Korea (Fig. 1), where the mid- to low region of the Nakdong River flows. The surface water area is 0.5 km2, and the average depth ranges from 0.6 to 1.4 m. The littoral area is characterized by macrophyte development from spring (May) to autumn (November). In the present study, we identified 8 species of macrophytes—
We investigated the species compositions of microcrustaceans and fish during spring (May), summer (August), and autumn (October) of 2012. Prior to collection of microcrustaceans, we considered habitat heterogeneity according to macrophyte presence. We created virtual grids over the map of the Jangcheok Lake and randomly selected 15 sampling points among the locations with macrophyte development. Using quadrat in each sampling point, we investigated macrophyte type. For microcrustacean collection and environment factors measurement, we collected water samples (10 L each) by using a 10-L column sampler at each sampling point. At a sampling point, we placed the sampler vertically into the water, in order to collect waster sample from the entire water column. A dissolved oxygen (DO) meter (Model 58; YSI Inc., Yellow Springs, OH, USA) was used to measure the water temperature and dissolved oxygen, and conductivity and pH were measured using a conductivity meter (Model 152; YSI Inc.) and Orion 250A pH meter (Orion Research Inc., Boston, MA, USA), respectively. The water samples were conveyed to the laboratory to measure the concentration of chlorophyll a and turbidity. Turbidity was measured by using a turbidimeter (Model 100B; HF Scientific Inc., Ft. Myers, FL, USA). The water samples were filtered through a Mixed Cellulose Ester (MCE) membrane filter (pore size, 0.45 μm) (A045A047A; Advantec MFS, Dublin, CA, USA), and the filtrate was used to determine the concentration of chlorophyll a based on Wetzel and Likens (2000). Microcrustaceans were collected through filtration of sampled water by using a plankton net (32-μm mesh), and the filtrate was preserved in formaldehyde (final concentration, approximately 5%). The microcrustaceans were identified and counted under an Axioskop 40 microscope (Zeiss, Oberkochen, Germary) at ×200 magnification, according to the classification key of Mizuno and Takahashi (1991).
After collection of microcrustaceans (i.e., approximately 30 min), we collected bluegill (
Results of physico-chemical parameters showed seasonal differences during study period. Water temperature exhibited the strongest seasonality; it was the highest in the summer and lowest in the winter. The percent (%) saturation of dissolved oxygen varied depending on temperature; summer and autumn showed lower saturation than spring and winter. The seasonal pattern of pH was also similar with % saturation of dissolved oxygen, lower in summer and autumn. Turbidity was the highest in autumn, but the average turbidity of each of the other seasons was similar. The chlorophyll a concentration was below 20 μg/L from spring to autumn and was high in winter.
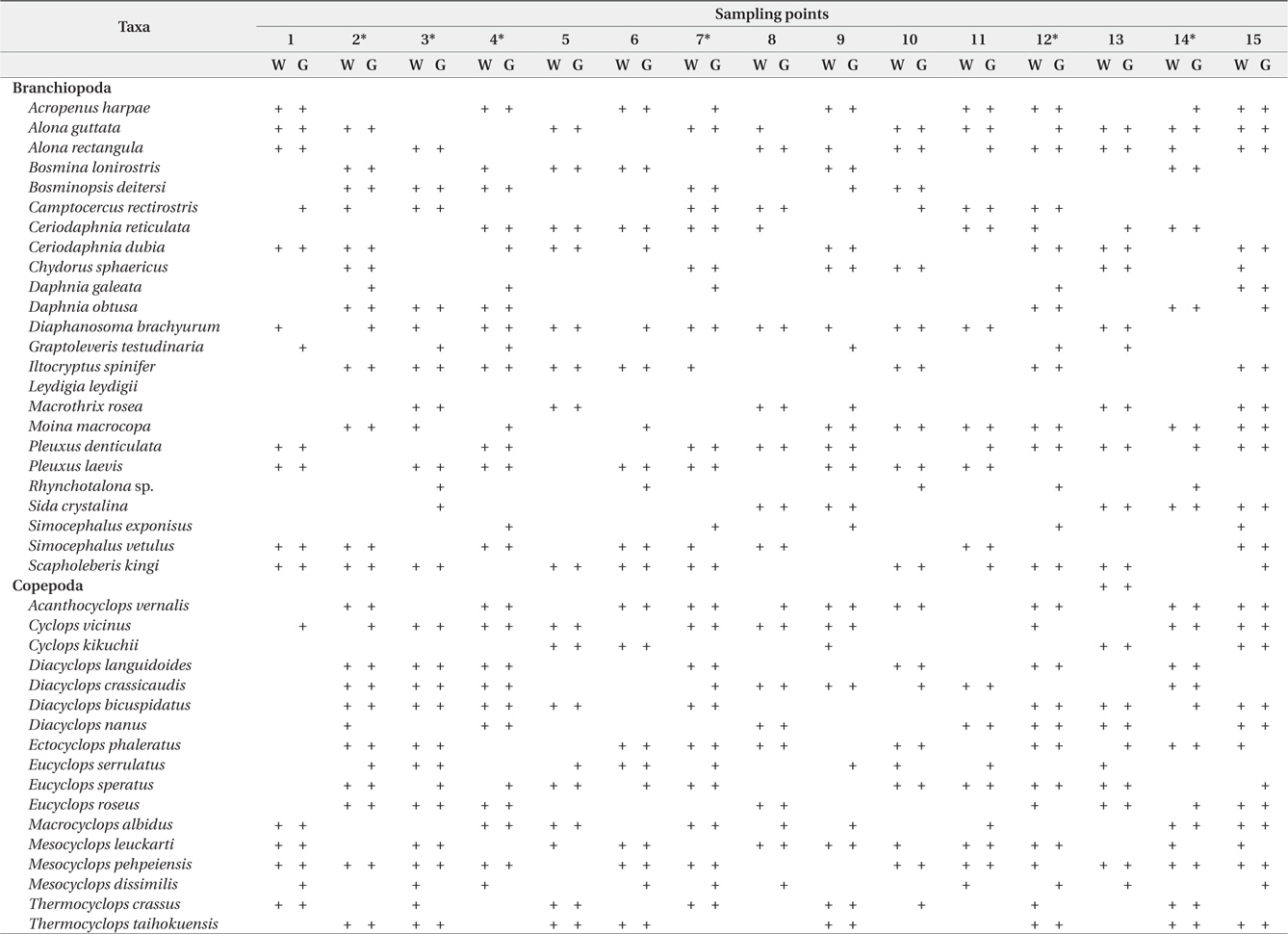
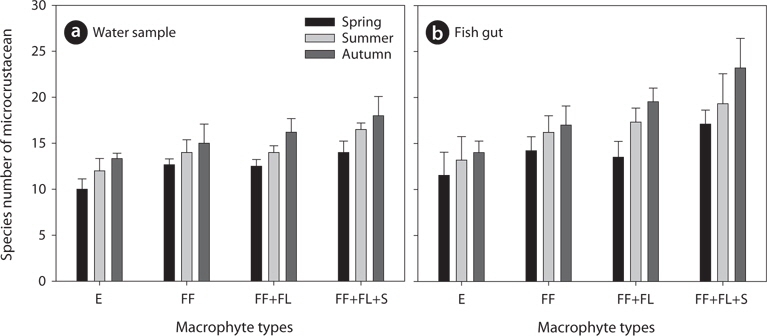
During the study period, we found a total of 41 microcrustacean species: 24 species belonged to Branchiopoda; the remaining 17 species, Copepoda. The microcrustaceans showed different species compositions between each sampling point (Table 1). The differences are interpreted as a result of heterogeneous structure by different macrophyte species. Macrophyte habitat structure is an important factor to determine microcrustacean species composition (Manatunge et al. 2000, Kuczyńska-Kippen and Nagengast 2006). Sakuma et al. (2002) observed different microcrustacean species compositions between reed and submerged macrophytes zones, caused by different morphological structures between the 2 macrophyte types. In comparison with other macrophytes, submerged macrophytes are known to contribute considerably to the formation of complex habitat structure in water and to support higher density and greater species diversity of microcrustaceans (Jeppesen et al. 1998, van Donk and van de Bund 2002). Therefore, in the present study, a high number of microcrustacean species at a sampling point may be explicable by the presence of submerged macrophytes (Fig 2) whereas relatively lower species number of microcrustacean at a sampling point may be explained by the absence of submerged macrophytes. In particular, emergent macrophytes supported lowest species number of microcrustacean. Although we observed a higher number of microcrustacean species in sampling point with submerged macrophytes, some species (
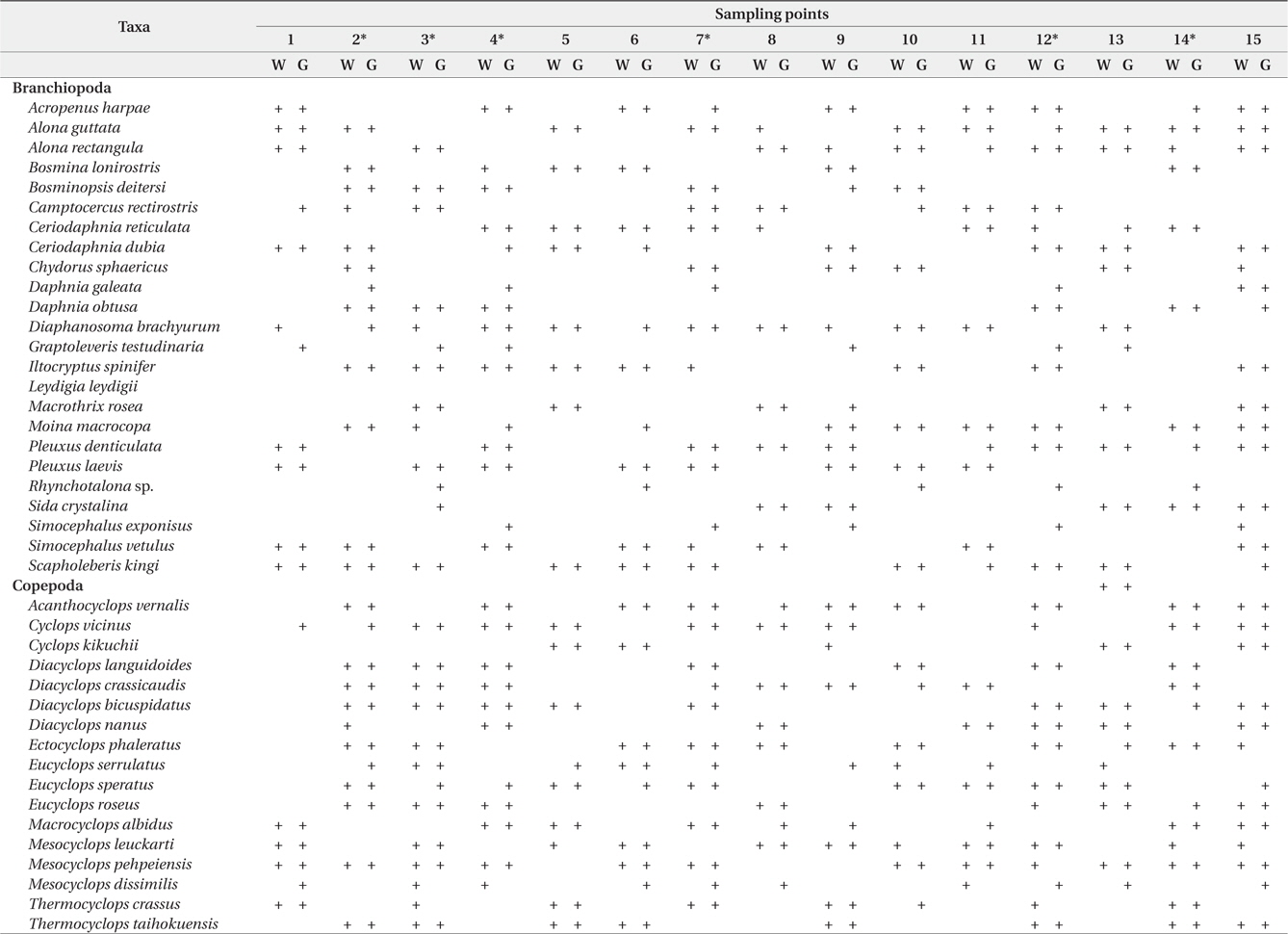
Microcrustacean species compositions in water sample and in the guts of fish collected from each sampling point
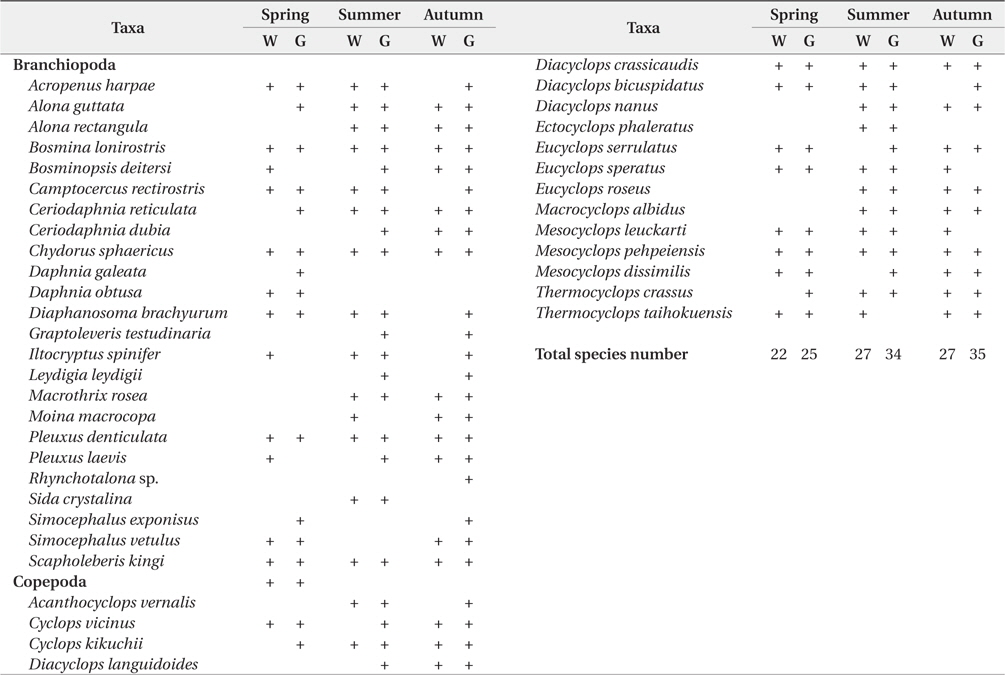
In the present study, we analyzed total 85 bluegill gut (spring, 24; summer, 32; autumn, 29). We found that the number of microcrustacean species was higher in bluegill guts than in the field (Table 1 and 2). The species number of microcrustacean was not significantly different between fish gut and water samples (Two-way ANOVA, df = 1,
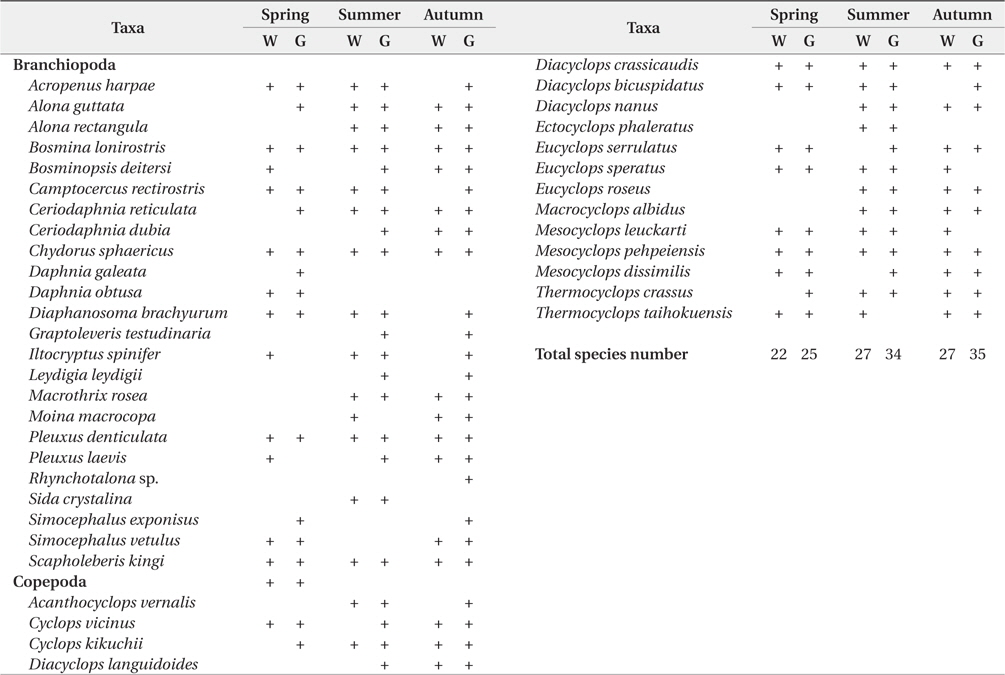
Species compositions of microcrustacean in water sample and in the fish guts in accordance with season
The results of our study verified the validity of fish gut analysis to monitor microcrustacean species compositions in ecosystems with excessive macrophyte development. The advantages of this method will be maximized when the habitat contains a large number of macrophytes because excessive macrophyte development frequently hinders the determination of microcrustacean species. In freshwater ecosystems, microcrustaceans are located at an intermediate level of the food web, where they play a key role in the transfer of energy and materials (Wetzel and Likens 2000). Thus, the use of a “biological monitoring agent,” such as bluegill gut analysis, will enable precise determination of species composition.