This study investigated the ability of Bothriochloa bladhii (Retz.) S.T. Blake (Poaceae), Cyperus ligularis L. (Cyperaceae), Commelina erecta L. (Commelinaceae), Mariscus umbellatus (Rottb.) Vahl (Cyperaceae), Fimbistylis miliacea L. (Cyperaceae) and Torulinium odoratum L. (Cyperaceae) to clean up various levels of used motor oil (UMO) contaminated soils. The plants were grown in 2 kg garden soils treated to 0%, 1%, 5% and 10% levels of UMO contamination. The plant growth parameters, chlorophyll contents and dry weight of test plants were measured. The phytoremediation ability of these test plants were assessed by measuring the uptake of hydrocarbons in terms of total hydrocarbon content (THC) as well as their percentage degradation values. There was significant (P < 0.05) reduction in leaf chlorophyll contents and dry weights of the test plant species planted in UMO contaminated soils. THC as well as the percentage uptake (or degradation) of hydrocarbons were both lowest in C. ligularis but highest in T. odoratum in all cases. The phytoremediation potential of test plants was highest in soils contaminated with 5% UMO. Based on the results of this study, all test plants with the exception of C. ligularis were potentially capable of undertaking phytoremediation. However, B. bladhii and T. odoratum proved most effective in the uptake and degradation of UMO.
The exponential increase in the number of automotive vehicles in Africa has concomitantly led to the extensive use of refined petroleum products. In particular, such vehicles are usually old and have low efficiency in utilizing the refined products. Ghana epitomizes this phenomenon. Activities of way-side private automobile mechanics are largely uncontrolled across the cities and towns in non-built up areas in Ghana. This, in turn, has led to rampant spillage and deliberate release of used motor oil (UMO) directly on the soil surface leading to potential contamination of groundwater. Used motor oil may contain heavy metals, such as lead, chromium, cadmium, arsenic, zinc and other materials like benzene, naphthalene, chlorinated hydrocarbons, and sulphur. One liter of oil can contaminate up to 1 million liters of water and can accumulate in the subsoil system, posing a risk to the environment. A single automotive oil change is estimated to produce 4–5 L of used oil (Singh et al. 2009). The amount of used motor oil disposed of improperly by auto mechanics every 18 days is approximately 42 million L. Approximately, 3.8 L (i.e., one gallon) of used motor oil that is re-refined will produce 2.4 L of lubricating oil, but it takes 159 L of crude oil to produce the same 2.4 L of lubricating oil. Obviously, these motor lubricants and UMOs do alter soil physical, chemical and microbial properties (Patrick-Iwuanyanwu et al. 2010) and inhibit plant growth (Okonokhua et al. 2007, Kayode et al. 2009). The hydrocarbon component of the oil is also known to interfere with integrity of membranes (Carson et al. 2006).
The ability of plants to thrive in oil contaminated media varies from one species to the other and this variation holds the key to the remediation of oil contaminated soils. To be a candidate for phytodegradation according to the Green Liver Model, 1) a suitable set of enzymes has to be present in the plant cells and 2) a soil contaminant has to be taken up by the plant through the xylem. As opposed to heavy metals that, as a result of their similarity to nutrients, are actively taken up by plant cells through transporter molecules (Krämer et al. 2007, Reichenauer and Germida 2008), no such specific transporter molecules exist in plants for organic contaminants of soil. These molecules are taken up passively by plant cells. Therefore, octanol– water partition coefficients (logKow) of such substances and their solubility in water, which facilitate the crossing of the outer cell membrane (the plasmalemma), are important and prerequisite for a substance to be taken up by plants (Reichenauer and Germida 2008). Substances with logKow between 0.5 and 3 are found to be taken up best by plants. Very hydrophilic substances (i.e., logKow < 0.5) can be transported well inside the plant but are not able to cross the plasmalemma, which is a lipid bilayer (Reichenauer and Germida 2008). Hydrophobic substances (i.e., logKow > 3), on the other hand, can be dissolved in the plasmalemma but are only poorly associated in the cytoplasm. For hydrophobic organic contaminants, including phenanthrene and pyrene, uptake by the roots of the plants shows a strong correlation with root lipid content (Gao et al. 2007). Both substances can be transferred from the root to the shoot, whereas no shoot-to-root transfer of these two polycyclic aromatic hydrocarbons (PAHs) in atmospheric deposition could be detected.
According to Garbisu and Alkorta (2001), there are two different strategies for phytoremediation of organics: direct phytoremediation and phytoremediation
In fact, existing research on phytoremediation has disproportionately focused on heavy metals. This study therefore, sought to determine the potential of some selected indigenous grass and grass-like species for phytoremediation of UMO contaminated soils. The specific objectives were to investigate the effect of the UMO on some growth parameters (chlorophyll concentration and biomass) of the selected plant species; measure the quantity of total hydrocarbon extracted by the grass species; and also to estimate the degradation ability of the species. It was envisaged that the plant species with the most proven potential could be employed in field trials.
>
Seedlings collection and identification
Seedlings of plant species used for this study were collected from various locations along the Kpone and Tema Newtown beaches and inland points within Ashaiman (05º42′00″ N, 00º01′60″ W) and Tema (05º40′ N, 00º00″ W), Ghana.
>
Site description and soil sampling
The soils (Alajo series) used in this study were collected from five profile pits located along the catena that runs from the Legon hill, in the northeastern direction. The study area lies within 05º38′00″ N to 05º42′00″ N, and 00º08′00″ W to 00º12′00″ W, approximately. This area is typical of the general ecology and geomorphology of the coastal savanna zone and the Accra Plains in general (Eze et al. 2010). The altitude of the study area ranged from 54 to 164 m above sea level. It experiences a mean annual rainfall of about 800 mm and mean annual temperature of 27ºC (Eze et al. 2010). The rainfall regime typically consists of two rainy seasons: a main wet season which falls from April to July, and a minor rainy season around September to mid November. This area is further characterized by a distinct dry season between December and February. Legon hill in the Accra Plains is an outlier of the Togo–Akwapim Range. The geology of the area is Togo (pre-cambrian) quartzite with smaller amounts of phyllite, sericite, schist, sandstone, and shale (Eze et al. 2010). The catena on the Legon hill under study consists of five soil series, comprising four Alfisols and one Vertisol (Eze et al. 2010).
>
Physical and chemical properties of soil
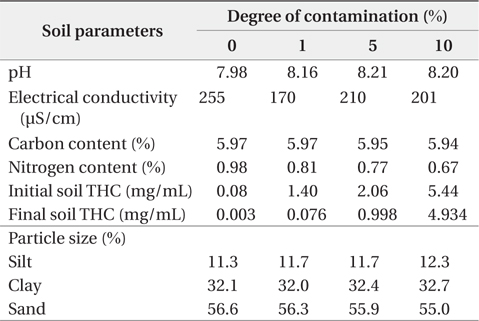
The textural characterization of the soils indicates they are predominantly sandy clay loam, sandy clay, and clay. Sand content of the soils varied from 250 to 790 g/kg and tended to decrease with depth, while the silt content ranged from 100 to 300 g/kg. Clay content ranged from 130 to 730 g/kg and generally increased with depth. Table 1 shows the physicochemical properties of soils used in this study. The Alajo series located at the bottom slope was predominantly clay with a clay content of ≥500 g/kg in all horizons. Elevation tended to influence the distribucoltion of clay and sand within the upper 0–30 cm depth of these soils. A decrease in elevation clearly indicated an increase in clay enrichment with a corresponding decrease in sand content, since the finer particles (clay fraction) are easily transported down to bottom slopes. The pH of the soils ranged from 5.0 to 7.9 (moderately acidic to slightly alkaline) and tended to decrease with depth. Soil electrical conductivity (EC) values ranged from150 to 300 µS/cm indicating that the soils were non-saline.
[Table 1.] Physicochemical properties of soil
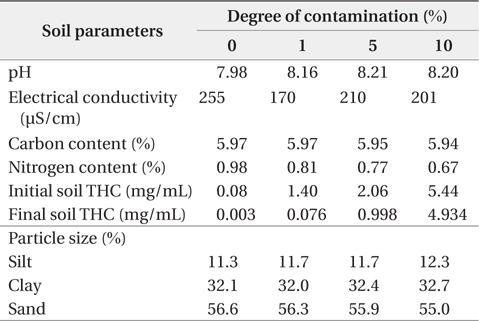
Physicochemical properties of soil
In this paper, degree of contamination is defined as the percentage weight of UMO contaminant with respect to dry weight of soil. The observed contamination level at some contaminated sites (i.e., environs of mechanical workshops) provided a basis for contamination of the soil in the laboratory at varying percentages. Based on this consideration, the soil was contaminated at 1%, 5%, and 10% degree of contamination for further studies. The initial degree of contamination was fixed at 1% because Ghana does not have any statutory percentage degree of soil contamination that it considers as hazardous waste.
The soil was obtained from the Experimental Farm site on the University of Ghana campus and prepared by sieving it through 2 mm mesh to exclude debris and spread out thinly in a rectangular tray to air dry. Two kilograms of the dried soil was treated by mixing thoroughly with 0 ml, 26 ml, 115 ml and 228 ml of UMO to obtain a control, 1%, 5% and 10% level of UMO contamination of the soil. The test plants were allowed to grow for 8 weeks in a greenhouse. Analyses were carried out 2, 4, 6, and 8 weeks after transplanting respectively. Soil pH was analyzed by Orion ion meter model 525A (Thermo Scientific, Beverly, MA, USA). Available nitrogen (%) and available phosphorus (%) were estimated by following the approach of Carter and Gregorich (2007), while total organic carbon was analyzed by following the method of Walkley (1947). Water holding capacity was measured hydrometrically.
>
Measurement of leaf chlorophyll
Leaf chlorophyll content of the entire test plants were analysed spectrometrically at 647 and 664 nm, using the Perkin-Elmer spectrophotometer (Lambda 20 VS VIS; Perkin-Elmer, Waltham, MA, USA) after extraction with 80% (v/v) toluene. The Witham et al. (1971) formula was used to determine the leaf chlorophyll content of the test plants. Total hydrocarbon content of the test plants was determined spectrometrically at 420 nm after extraction with toluene 80% (v/v) and degradation ability of the test plants was estimated as the amount of hydrocarbons removed from the contaminated soils in relation to the amount applied (Merkl 2005). There were 12 replicates of each selected plant species per treatment option.
Used motor oil (UMO) was chosen as the contaminant for this study. It was obtained from a local automobile workshop. The grade of used oil was the Society of Automotive Engineers (SAE) 10W. This means that the oil can still be pumped by the engine at a temperature as low as minus 10 degrees during winter. Physical properties of the UMO are shown in Table 2. The hydrocarbon components of the UMO consisted of saturates (76%), aromatics (12%), polar compounds (3%), asphaltenes (1.5%), volatile compounds (3.5%) and other components not characterized (4%). The metal components of the UMO consisted of Al (12 mg/kg), Sb (612 mg/kg), Ba (7,512 mg/kg), Ca (38,012 mg/kg), Cu (1,312 mg/kg), Fe (13,812 mg/kg), Pb (45,012 mg/kg), Zn (68,012 mg/kg), Mg (20,012 mg/kg) and Si (1,912 mg/kg).
[Table 2.] Physical properties of used motor oil (UMO)

Physical properties of used motor oil (UMO)
The calculated weight of UMO to achieve the desired degree of contamination was sprayed on 2 kg of each type of soil. The soil–contaminant mixture was pulverized manually and thoroughly mixed for 1 h in a covered tray. The mixture was placed in a covered container for 1 week so that the UMO would come to equilibrium with the soils. During this period, the soils were mixed periodically. Dry soils required 24 to 48 h to come to equilibrium with water (ASTM 1993).
Total hydrocarbon uptake indicates the ability of total hydrocarbon accumulation in different plant parts of collected species. Concentration ratio for total hydrocarbon was calculated as per the following formula: CR = (shoot total hydrocarbon concentration)/(root total hydrocarbon concentration). Thus, in roots it is an index of translocation. Generally, the CR in all confirmed hyper-accumulators of hydrocarbons are >1, whereas it is usually <1 in non-accumulators.
The statistical package for the social sciences (SPSS) ver. 16.0 for Windows software (SPSS Inc., Chicago, IL, USA) was used to analyze the means of data obtained as well as to display them graphically. The means were compared using two-way analysis of variance (ANOVA), significance was declared for values of
>
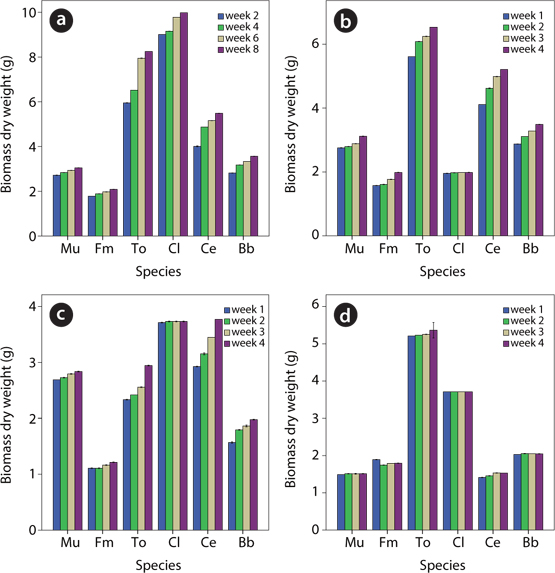
Growth of plant species in contaminated soil
Test plants thrived in all the levels of soil contamination with UMO except
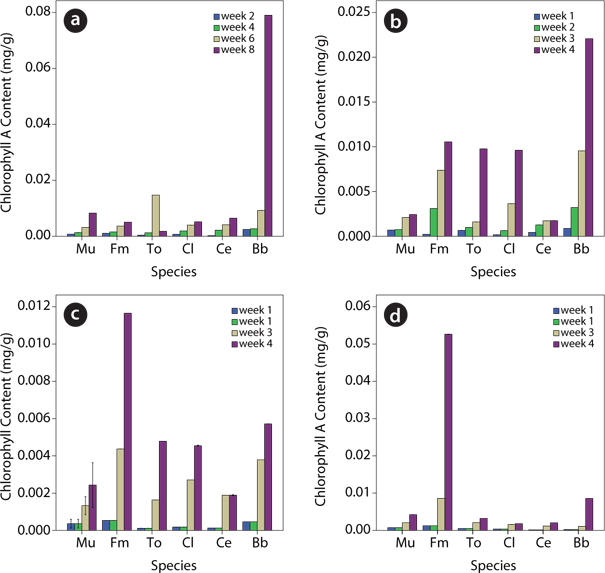
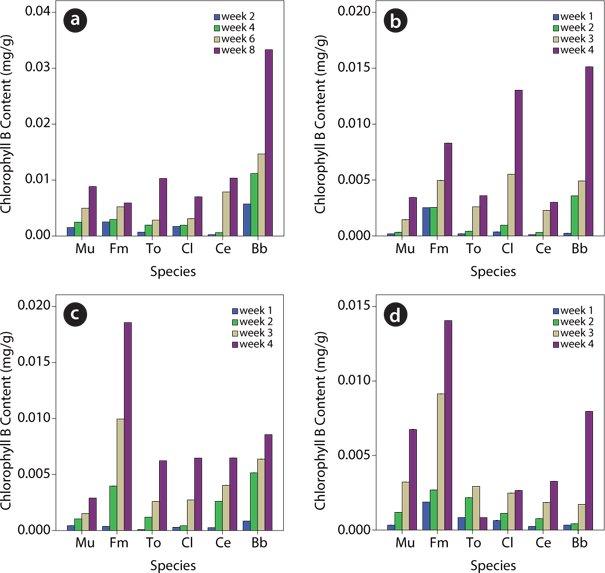
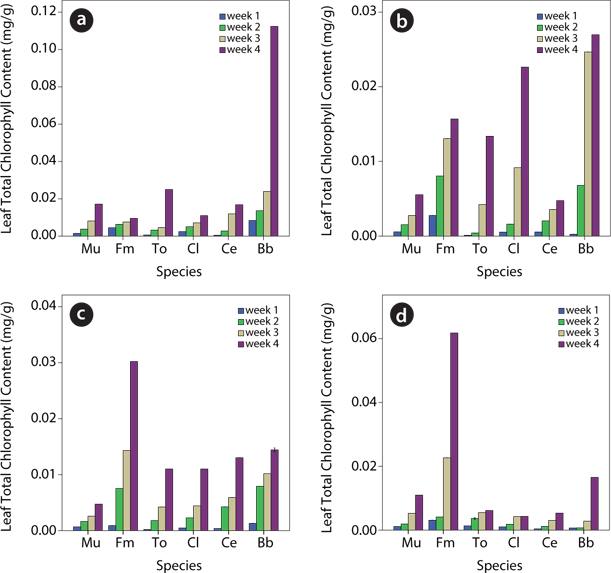
Cholorophyll content of plants species in contaminated soil
Data on chlorophyll content showed that the leaf chlorophyll content (A, B and total chlorophyll) of test plants within each treatment level generally increased from week 1 to week 4 (Figs. 3-5). For chlorophyll A,
>
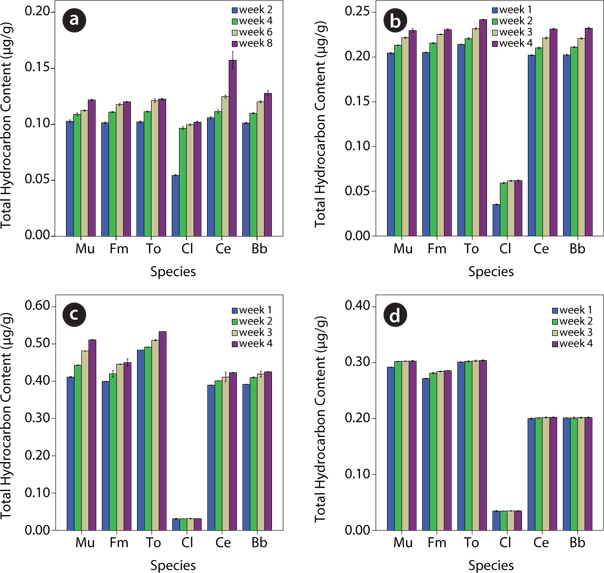
Hydrocarbon content of the plants species in contaminated soil
There was a steady increase in hydrocarbon content of test plant species from week 1 to week 4 of the 0%, 1% and 5% levels (Fig. 6). For
>
Percentage degradation of hydrocarbons in contaminated soil
The percentage degradation of hydrocarbons varied with the various treatments and plant species used as depicted in Fig. 7. The general degradation was slightly above average (i.e., 50%) as the highest recorded was 52.7% for
>
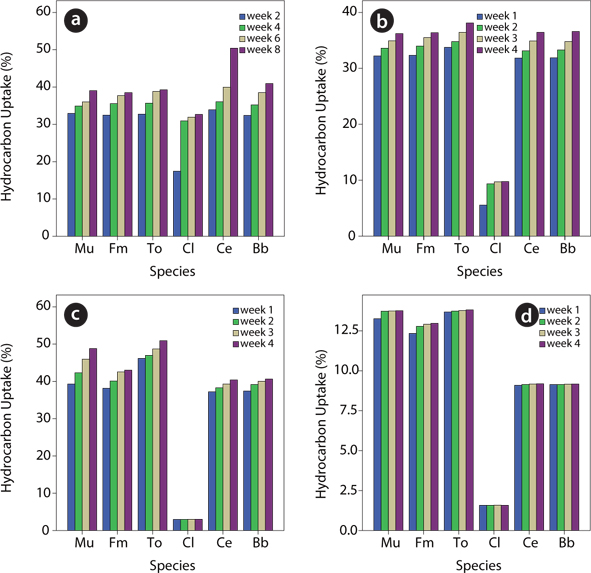
Species-specific hydrocarbon uptake
The treatment options had significant effect on the uptake of hydrocarbons in the various plant species. For
Species variations in soil–plant transfer (concentration ratios) were observed and the magnitude of uptake for the plant species in decreasing order was as follows:
Five out of the six plants investigated in this study showed a great ability to survive in UMO contaminated soils as demonstrated by their growth monitoring data. The exception was
The data obtained showed that within a given treatment level, leaf total chlorophyll as well as the chlorophyll A and B of the test plants were found to increase from week 1 to week 4. However, upon comparison of the different treatment levels, species response to the effect of the various levels of the UMO contamination in the soils was found to be significantly (
The observation made in this study that the total hydrocarbon content of test plants varied from species to species and increased throughout the study period in all except for one specie is consistent with the findings of Palmroth et al. (2002) and Ogbo et al. (2009) who, in similar but separate studies using different plant species reported variations in the hydrocarbon contents of the test plants used. It is thus, apparent that different plant species have different potentials for the uptake of hydrocarbons. Frick et al. (1999) explained that the ability of plants to conduct phytoremediation depended on the activities of the degrading microorganisms in the rhizosphere of these plants which secreted chemical substances into the rhizosphere that aided plant roots to absorb hydrocarbons into their tissues. Thus, genetic makeup of the species and micro-environmental factors of the rhizoplane and rhizosphere could influence the ability of the plant species to carry out phytoremediation, causing them to respond differently to the toxicity posed by the oil (Spiares et al. 2001).
This study also revealed that the 5% UMO contaminated soils recorded the highest hydrocarbon uptake and degradation values compared to the other treatment options. This shows that there is an optimum level or threshold of the contaminant that can sustain the growth of the plant species even though the growth realized was lower than in the case of the uncontaminated soils. The degradation ability of the test plants was appreciable though the experiment was conducted in unamended soils. The degradation values attained by
This study set out to investigate the ability of six tropical grass species namely