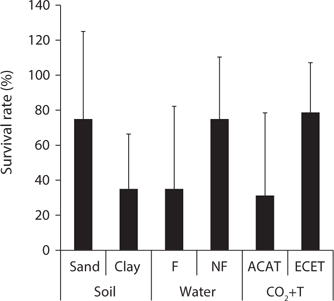
Mankyua chejuense is a native endangered plant distributed only in Gotzawal, a forested wetland, in Jeju Province, Korea. In order to determine the optimal environmental conditions for the growth and development of M. chejuense, we investigated the above- and below-ground growth responses and survival rate to various soil texture (sand and clay), water regimes (flooding and non-flooding), and CO2+T (ambient and elevated) conditions. All of the treatments had significant effects on aboveground growth parameters, while only the water regime and CO2+T treatments influenced belowground growth. The survival rate of M. chejuense was about twice higher under the sand, non-flooding and elevated CO2+T conditions than clay, flooding and ambient CO2+T conditions. These results indicate that M. chejuense grows in well-drained sandy soil conditions and elevated CO2 concentration and temperature situations. Thus, there is a need to maintain M. chejuense under constant non-flooding soil conditions by implementing appropriate soil drainage strategies.
The distribution of
Rare and endangered species with limited climatic ranges or restricted habitat requirements are generally less tolerant to environmental stresses and have lower phenotypic plasticity during acclimation to a broader range of environmental conditions than widely-distributed species (Sakai et al. 2001, Parmesan and Yohe 2003, Baruch and Jackson 2005). Thus, to understand any plant, it is important to obtain basic ecological information regarding the population response to diverse environmental factors such as soil, water and temperature by conducting detailed field- and laboratory-based ecological and physiological measurements (Jeffries 2006).
Recently, Kimball et al. (1993) demonstrated that elevated CO2 concentrations and increased temperatures have had profound effects on plant life history. Native species specific to local regions may be negatively affected than widespread species under global warming (Song et al. 2009), and thus at greater risk of extinction. However, there has been attempt to elucidate the growth response or belowground dynamics of this plant to environmental gradient conditions, despite the fact that this information is essential to plant population conservation and habitat management (You and Kim 2010).
In the present study, we measured the survival rate, and aboveground- and belowground growth under various soil textures, water regimes, CO2 concentration and temperature to verify the growth response of above- and below-ground parts of
The soil texture treatments were sand (1-2 mm particle size), or clay (particle size < 0.2 mm) which consisted of vermiculite. The water regimes were flooding (maintenance of water at 1-2 cm above the root), and nonflooding, which were achieved by pouring water into the growth pots and releasing it through holes in the bottom.
Global warming treatment was ambient CO2 and ambient temperature (ACAT) and elevated CO2 and elevated temperature (ECET). In the ACAT, the air is maintained at the ambient CO2 concentration and temperature of the immediately surrounding air, which averages about 370- 380 ppm on a 24-hours basis. The ECET was maintained by inputting a small quantity of pure CO2 through two perforated plastic hoses so as to maintain the CO2 concentration at approximately 742.30 ± 16.92 ppm, which is twice that of the ambient (360.38 ± 9.19 ppm) concentration. CO2 concentration was monitored by a TEL-7001 CO2 sensor (Onset Computer, Bourne, MA, USA) at 30-min intervals, and data was stored on HOBO U12 datalogger (Onset Computer, Bourne, MA, USA) to evaluate the stability of the CO2 concentration in the treatment. According to IPCC scenario (2007), the atmospheric CO2 concentration is predicted to be double within the next 100 years.
The mean temperature in the treatment was about 2.5ºC higher than the control. The air temperature was measured using a TR-71U thermo recorder (T&D Co., Matsumoto, Japan) at the same height in the control and treatment greenhouses during the study period. This study was conducted in glass greenhouse of 12 m × 7.8 m × 5 m (length × width × height). The floor surface area of each control and treatment is 46.8 m2.
Four individual ramets of
At the end of the study in November 2011, we counted the number of shoot, leaves, sporophyll and measured the length of shoot and sporophyll. Belowground growth of
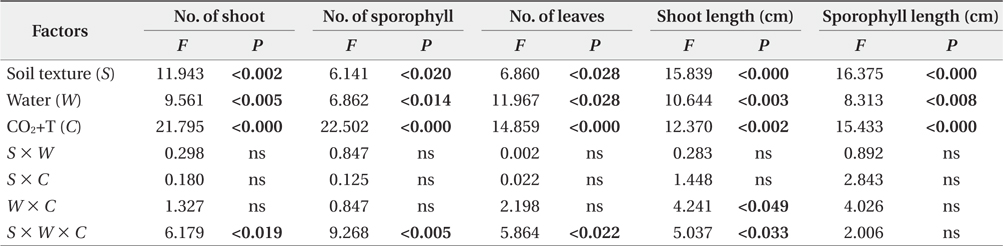
The effects of soil texture, water and elevated CO2+T on the aboveground and belowground growth parameters of
The multivariate analysis of variance (MANOVA) is a rhicomplex statistic similar to ANOVA but with multiple dependent variables analyzed together. That is, the MANOVA is a multivariate extension of ANOVA (Zar 2009). MANOVA was used to analyze the effects of soil texture, water and elevated CO2+T and their interaction on aboveand below-ground growth of
Plant growth responds to multiple environmental factors, light, water, temperature and soil properties, also two or more resources simultaneously limit plant development (Chapin et al. 1987). Unfortunately for most endangered species, little information is available on their ecological requirements. Thus, it is necessary to determine optimal environmental conditions of species for the conservation of rare and endangered plants (Smith et al. 1993). According to Lee et al. (2012), the habitats of
As a result, the pooled data of the
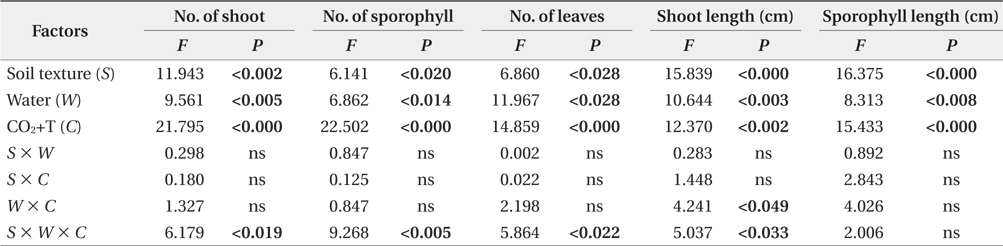
Effects of environmental factors and their interactions on aboveground growth of Mankyua chejuense
The average aboveground growth response for
Growth and potential reproduction in pteridophyte are reflected in the number of leaves that are produced within a given time period (Sharpe and Mehltreter 2010). In the present study, there were significant water and CO2+T effects on number of leaves (Fig. 1e). The number of leaves of
Many pteridophytes supplement their sexual cycles with various forms of vegetative reproduction (Yatskievych 2003). The branching of the rhizome and formation of adventitious buds on rhizomes is ferns of Ophioglossaceae have been reported (McMaster 1996). According to Kim (2004),
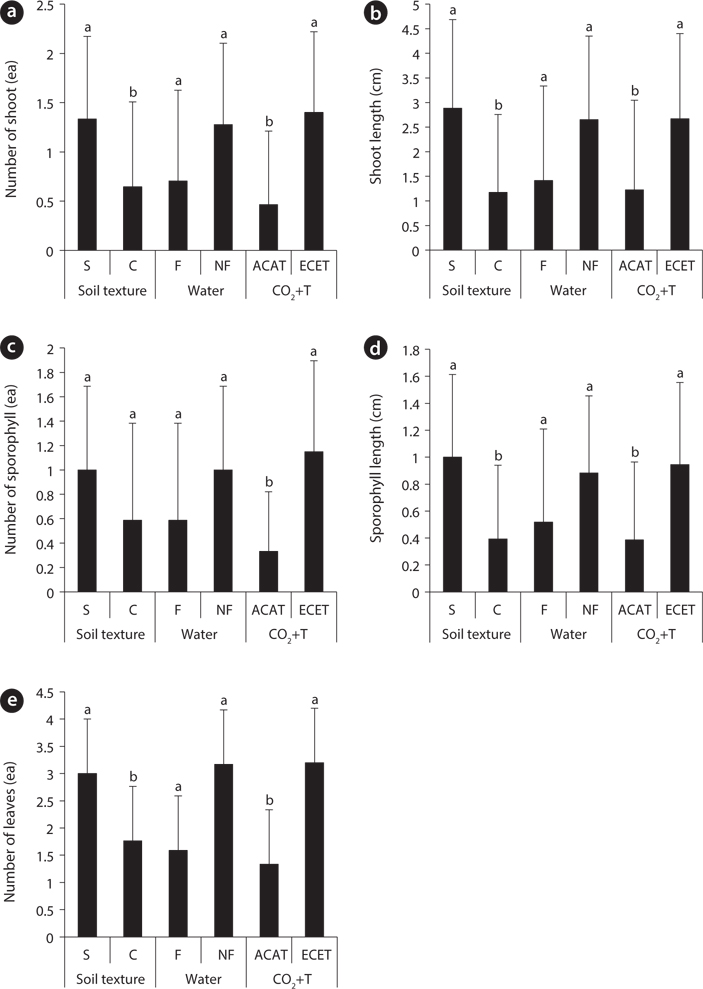
Unlike aboveground growth responses to three environmental factors, the development and growth of aboveground of
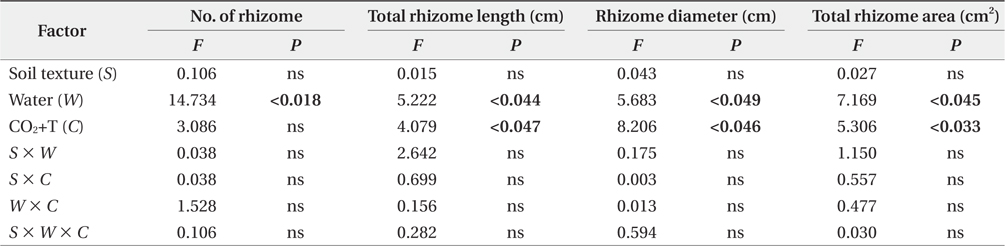
Effects of environmental factors and their interactions on aboveground growth of Mankyua chejuense
The growth increment of the number and total area of rhizome showed similar trends (Fig. 2a and Fig. 2d) between 2009 and 2011. The mean number and total area of rhizome increment in the non-flooding treatment were 3.6- and 9.8-fold higher than in the flooding treatment respectively. In the CO2 and temperature treatments, the growth increments in number and total area of rhizome were approximately 2.7- and 4.2-fold higher in the ACAT than in the ECET. However, soil texture effects were not detected in the growth increment of number and total area of rhizome.
Elevated CO2 concentration and temperature stimulated rhizome growth for
We found that there was a significant effect of water regimes and elevated CO2 and temperature on the aboveand below-ground growth of
Scenarios for future global and regional climate change will include elevated atmospheric CO2 (Jones 2000), warmer average air temperatures and increasing the frequency of severe weather events, such as flooding (IPCC 2007). Under future climate change, the growth and survival of