The effectiveness of N-acetyl-L-cysteine (NAC) to protect blood cells against Mitomycin C (MMC) induced genotoxicity was investigated in human chronic myeloid leukemia cells (KCL22) using the alkaline comet assay. The comet assay was selected as sensitive and rapid method for analysis of DNA damage and repair in individual cells. NAC treatment alone did not produce any damage in KCL22 cell. But NAC was found to be effective in reducing genotoxic damage in KCL22 cells exposed to MMC. These results confirm the literature data that, given the safety and ability to reduce DNA damage. NAC may be useful to prevent drug-mediated genotoxicity.
The chemotherapeutic agents usually exert severe side toxic effects. The use of some pharmacological and dietary compounds to protect organs that are not targets in association with chemotherapy has been encouraged. Mitomycin C (MMC) has a wide clinical antitumor spectrum with efficacy in various tumor types. Metabolism of MMC leads to generation of mono- and bi-functional alkylating species and/or reactive oxygen species (ROS). DNA alkylation results in both inter- and intra-strand cross-linking of two nucleic acid chains and linking nucleic acid to protein, which could be a major factor for the disruption of nucleic acid functions (Tomasz 1995, Pawar et al. 2009).
The protective features (related to antioxidant activity) of
MMC has been widely used against various solid tumours. Thus, strategies for minimizing the toxicity of MMC as well as other antitumor drugs using antioxidant and antigenotoxic compounds are of clinical interest. Earlier the possibility to protect against MMC-induced oxidative stress was shown in Vero cells (Rjiba-Touati et al. 2013).
Blood cells are particularly susceptible to the effects of chemotherapy. Because of this, in the present study, we evaluate effectiveness of NAC to protect blood cells against MMC genotoxicity using the alkaline comet assay in human chronic myeloid leukemia cells (KCL22).
>
Cell line incubation and treatment
The cell line KCL22 (i.e., suspension cell line of human chronic myeloid leukemia in blast crisis) provided by Dr. T. Liehr (Institute of Human Genetics, Germany) was used in the experiment. Cells were routinely maintained in the growth medium RPMI-1640 supplemented with 10% fetal bovine serum (Sigma-Aldrich, Seelze, Germany; Biochrom AG, Berlin, Germany) at 37˚C. Cells were seeded into 15 mL glass vials at the concentration 0.5 × 106 cell/mL (2 mL of cell suspension per vial) and incubated for 24 h. NAC solved in water was added to the cell cultures at the final concentrations 100, 500 and 1000 μM for 24 h. The concentrations of NAC were chosen on the basis of the literature data and our preliminary experiments. After incubation with NAC cells were treated with MMC (1 μg/mL) for 2 h. Concentration and time treatment were determined in our preliminary experiments as optimal to induce moderate level of DNA damage in KCL22 cells.
The level of DNA damage was evaluated by standard alkaline single-cell gel electrophoresis (comet assay) (Tice et al. 2000). 20 μL of cell suspensions mixed with 0.5% low-melting agarose (80 μL) were added to slides precoated with 1% normal-melting agarose. After the solidification of gel layer the slides were immersed in a lysis solution (2.5 M NaCl, 100 mM EDTA disodium salt (pH 8.0), 10 mM Tris buffer (pH 10.0) and 1% TritonX-100) at 4˚C for 60 min. Slides were placed in electrophoresis buffer (0.3 M NaOH, 1 mM Na2EDTA, pH 13) to allow DNA to unwind. Electrophoresis was performed for 20 min at 300 mA and 1 V/cm. Slides were neutralized with Tris-HCl buffer, pH 7.5, and stained with 20 μg/mL ethidium-bromide for 10 min.
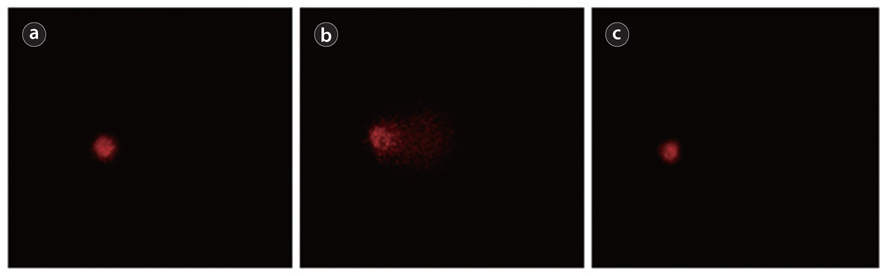
Slides were examined at ×250 magnifcation on an Axiophot fluorescent microscope (Zeiss, Oberkochen, Germany). At least 150 cells were scored per animal (50 cells scored per each of three replicate slides). Images of comets were recorded with a Variocam video camera with high sensitivity (PCO, Kelheim, Germany) and processed on a computer program Comet Assay IV ver. 4.3 (Perceptive Instruments, Bury St Edmunds, UK). Tail moment and tail intensity are used to evaluate the extent of DNA damage.
Statistical analysis of the results was performed using SPSS ver. 19 (SPSS Inc., Chicago, IL, USA) with application of non-parametric Mann-Whitney test (U test).
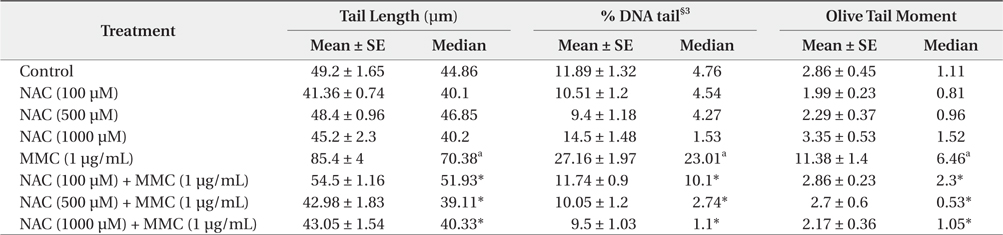
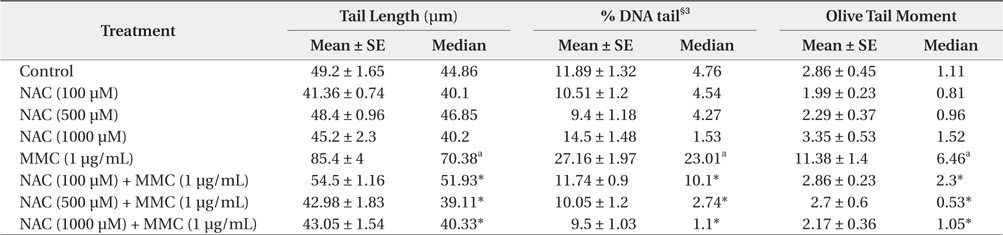
The various parameters of the comet assay in KCL22 cells treated with NAC and MMC are presented in Table 1. The observed tail length (TL), Olive tail moment (OTM) and percentage DNA in tails (% DNA tail) in MMC-treated cells were significantly (
[Table 1.] Protective effect of NAC§1 against MMC§2-induced DNA damage in the KCL22 cells
Protective effect of NAC§1 against MMC§2-induced DNA damage in the KCL22 cells
All of the tested doses of NAC were effective in exerting significant protection against the genotoxicity of MMC as evident from a reduction in the observed TL, OTM and % DNA tail, compared to the positive control. With increasing concentration of NAC from 100 to 500 μM, protective effect is essentially enhanced. NAC at 1000 μM induced further decrease of % DNA tail and minor increase in TL and OTM. Thus, the concentration of NAC 500 μM can be considered as the most effective.
NAC treatment alone did not produce any damage to the KCL22 cell at doses investigated.
Findings from these studies demonstrated that under this condition, NAC was found to be effective in reducing genotoxic damage in KCL22 cells exposed to MMC.
This study evaluated the protective effects of NAC on DNA damage induced by MMC by means of the single-cell gel electrophoresis (comet) assays. Pre-treatment of NAC at the tested doses significantly reduced the DNA damage induced by MMC in KCL22 cells as indicated by the decrease in the comet parameters TL, OTM and % DNA tail.
The comet assay was selected for our study as sensitive and rapid method for analysis of DNA damage and repair in individual cells. For the realization of this method cells are embedded in a thin layer of agarose on a glass slide, and then lysed. Thus membranes and soluble cell constituents, as well as histones, are removed, leaving the DNA still super coiled and attached to the nuclear matrix. Subsequent alkaline incubation and electrophoresis causes DNA loops containing breaks to move towards the anode, forming a ‘comet tail’ when visualized by fluorescence microscopy. The images resemble comets, and the relative content of DNA in the tail indicates the frequency of breaks (Dusinska and Collins 2008). The simple principle of DNA breaks detection makes it a good technique for studying of cellular responses to genotoxic and antigenotoxic agents in vitro and in vivo (Pawar et al. 2009, Furtado et al. 2010, Erdem et al. 2012).
MMC mainly induces DNA-DNA interstrand cross-links. However, an increase in DNA migration in the comet assay was shown in human blood cells (Pfuhler and Wolf 1996). This result may be explained by the ability of MMC to form hydrogen peroxide and hydroxyl radicals after reductive bioactivation (Dorr 1988). The comet assay detects H2O2-induced DNA breaks with high sensitivity (Fairbairn et al. 1995), even a small amount of H2O2 being sufficient for the induction of measurable DNA damage. Therefore, the protective effect of NAC against MMC can be attributed to its ability to scavenge the reactive oxygen species, hence preventing direct mutagenicity and DNA damages (Yedjou et al. 2010).
NAC has been shown to exert protective effects in a variety of experimental test systems. It is particularly important that NAC displays an ability to protect against DNA damage and in the same time was not effective in protection against cytotoxicity in different test-systems (Lewerenz et al. 2003, Reliene et al. 2009, Kim et al. 2013). This ability of NAC is very promising to decrease severe genotoxic side effect of antitumor drugs in normal cells without reduction of their treatment efficiency, mainly based on cytotoxic activity. Given these properties, NAC has the potential to be used as a selective protector against genotoxicity in normal tissue during radiotherapy or chemotherapy.
In conclusion, we have demonstrated that NAC supplement may effectively diminish MMC-induced DNA damage in KCL22 cells, possibly by scavenging ROS induced by MMC. Also, the protective action of NAC through other mechanisms is not excluded. These results confirm the literature data that, given the safety and efficacy of NAC, it may be useful to prevent drug-mediated genotoxicity.