We estimated the influence of environmental factors on zooplankton communities at 25 reservoirs during winter (December 2010 to January 2011). Among zooplankton groups, Cyclops vicinus is more dominant during winter, and this is positively related to withered vegetation area and dissolved oxygen level. Therefore, the presence of withered vegetation might be considered as an important factor to determine C. vicinus distribution during winter. We considered that withered vegetation might be utilized as a habitat for C. vicinus, as well as provide an attachment substrate for periphytic algae. Abundance of periphytic algae can lead to high concentration of dissolved oxygen. Although copepods prefer high water temperatures for increasing their population growth, if Cyclops can overcome low temperature stress that leads to disruption of population, their population growth initiation in the next growing season (i.e. next spring) is possibly propelled by the winter population.
Zooplankton distribution is strongly influenced by water body environmental variables, such as abiotic factors (e.g., water temperature, salinity, stratification, and advection), biotic factors (e.g., food limitation, predation, and competition), or a combination of both (Roff et al. 1988, Escribano and Hidalgo 2000, Beyst et al. 2001). Therefore, recognition of environmental variables in the distribution of zooplankton has been central to limnological research. In temperate regions, environmental factors in water bodies change dramatically according to the season, which determines the seasonal ontogeny and population growth of zooplankton (Marneffe et al. 1996).
Among diverse environmental factors, water temperature is clearly altered by seasonality, and controls metabolic rates and circadian rhythms, such as mating and dormancy (Hirche 1987, Robinson et al. 1983). Low water temperatures decrease the metabolism of zooplankton, which reduces their life cycle and feeding. The aforementioned circumstances affect their predators (mainly fish) as well (Brandt 1993), and hence, winter is believed to be a unique period during which zooplankton are not interrupted by predators. Therefore, we hypothesize that zooplankton communities in winter are affected by different environmental variables when compared with other seasons (spring, summer, and autumn). In particular, withered vegetation does not reduce phytoplankton biomass through shading effects (Sand‐Jensen and Søndergaard 1981) and allelopathy (van Donk and van de Bund 2002), and should thus support a large abundance of not only food sources, but also zooplankton. Unfortunately, distribution patterns of zooplankton related to environmental variables during winter have been insufficiently studied.
The primary objective of our study was to investigate the distribution pattern of zooplankton during winter. We investigated the following: (1) the influence of diverse environmental variables on zooplankton distribution, and (2) the contribution of withered vegetation to support a large abundance of zooplankton.
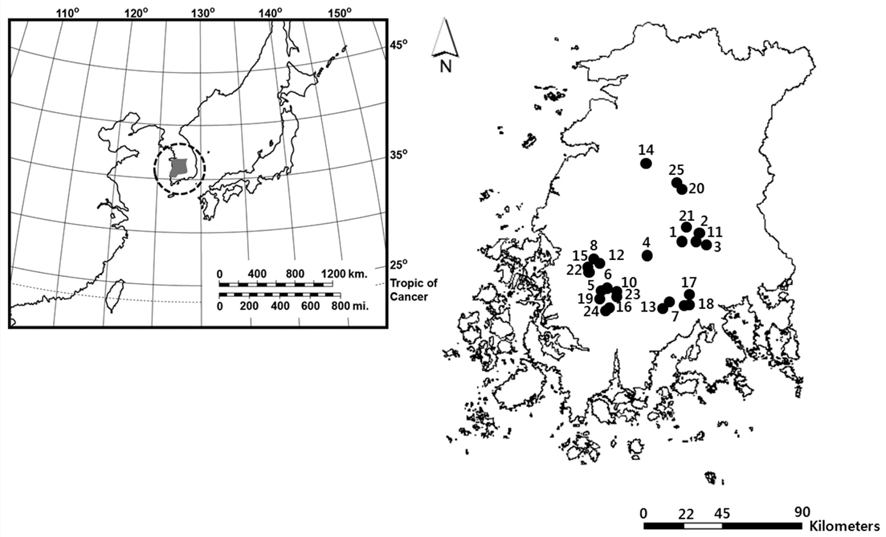
South Korea is located in East Asia, and possesses a temperate climate. Four distinct seasons lead to a dynamic succession of the biological community in Korean freshwater ecosystems. In particular, this country experiences an average temperature of −0.8°C during winter (from December to January). The reservoirs monitored in this study are located in southwestern Korea, within the central and lower reaches of the Yeongsan River (Fig. 1). We investigated environmental parameters (reservoir area, withered vegetation area, water temperature, dissolved oxygen, and chlorophyll a) and zooplankton communities in 25 reservoirs during winter (December 2010 to January 2011). We established three sampling points in littoral zone of each reservoir. The sampling points were randomly selected based on virtual grids constructed over maps of the reservoirs. We used a YSI 58 dissolved oxygen (DO) meter (YSI Inc., Yellow Springs, OH, USA) to detect water temperature and DO (% saturation). Chlorophyll
For zooplankton collection, we collected 5 L water samples using a 10 L column sampler at each sampling point. The sampled water was filtered through a plankton net (32-μm mesh net), and the filtrate was preserved in formaldehyde (final concentration: 5%). The zooplankton were identified and counted using an Axioskop microscope 40 (Carl Zeiss, Oberkohen, Germary) at ×200 magnification, based on the classification key published by Mizuno and Takahashi (1999).
Canonical correspondence analysis (CCA) was used to identify the relationship between the zooplankton communities and environmental parameters by using Canoco ver. 4.5 (Ter Braak and Smilauer 1998).
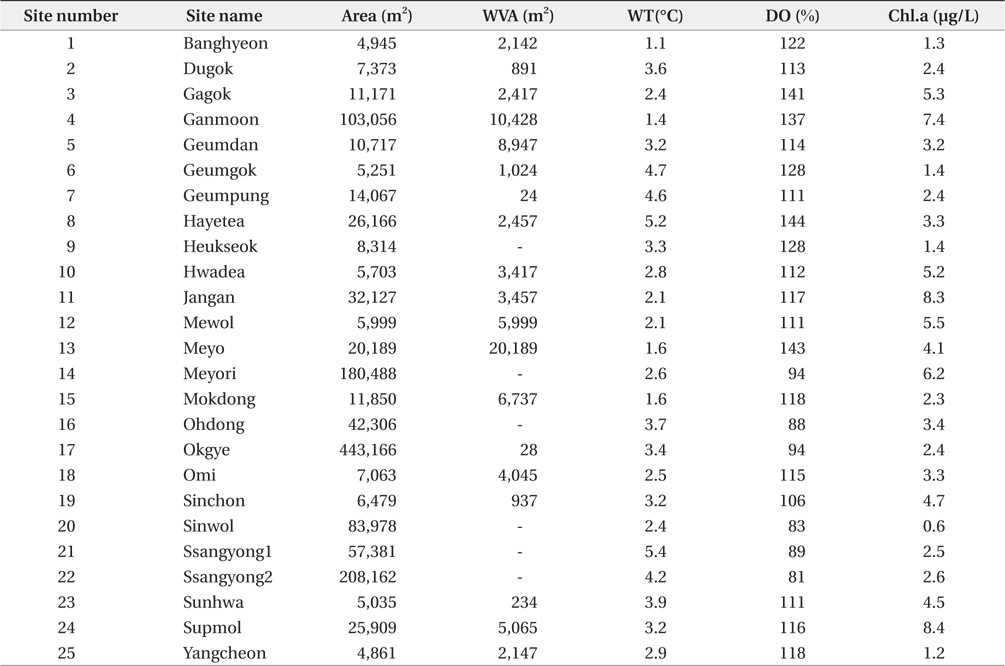
The observed levels for environmental factors (reservoir area, withered vegetation area, water temperature, dissolved oxygen level, and chlorophyll
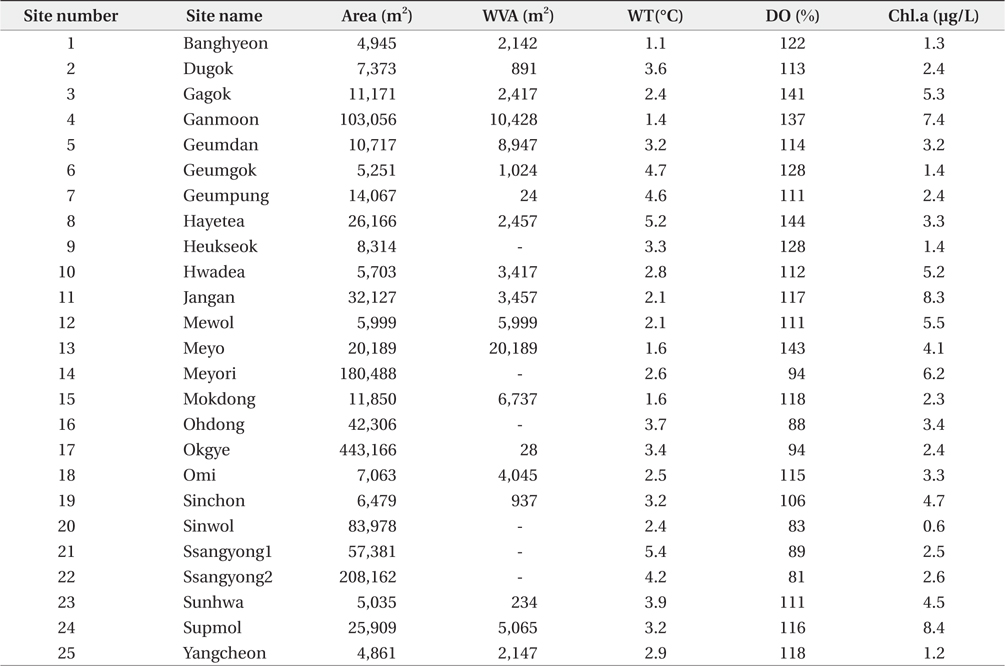
Environmental parameters measured at study sites during winter. WVA, withered vegetation area; WT, water temperature; DO, dissolved oxygen; Chl.a, chlorophyll a
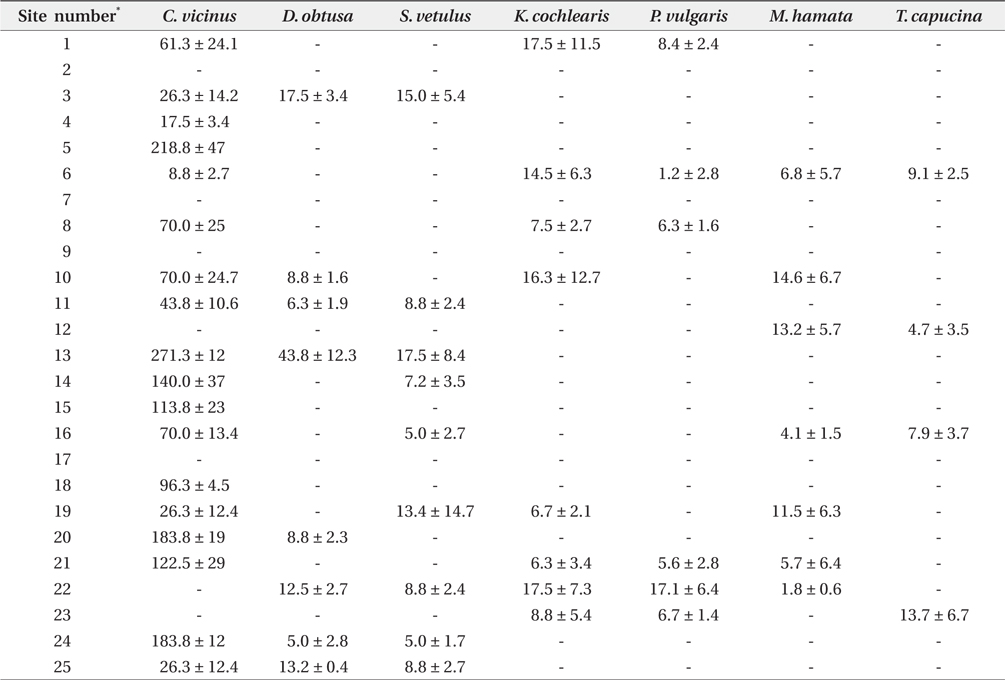
Zooplankton were collected across all investigated coreservoirs, but no zooplankton was found in the sites 2, 7, 9, and 17 (Table 2).
[Table 2.] Zooplankton species density (individual/L) at study sites during winter
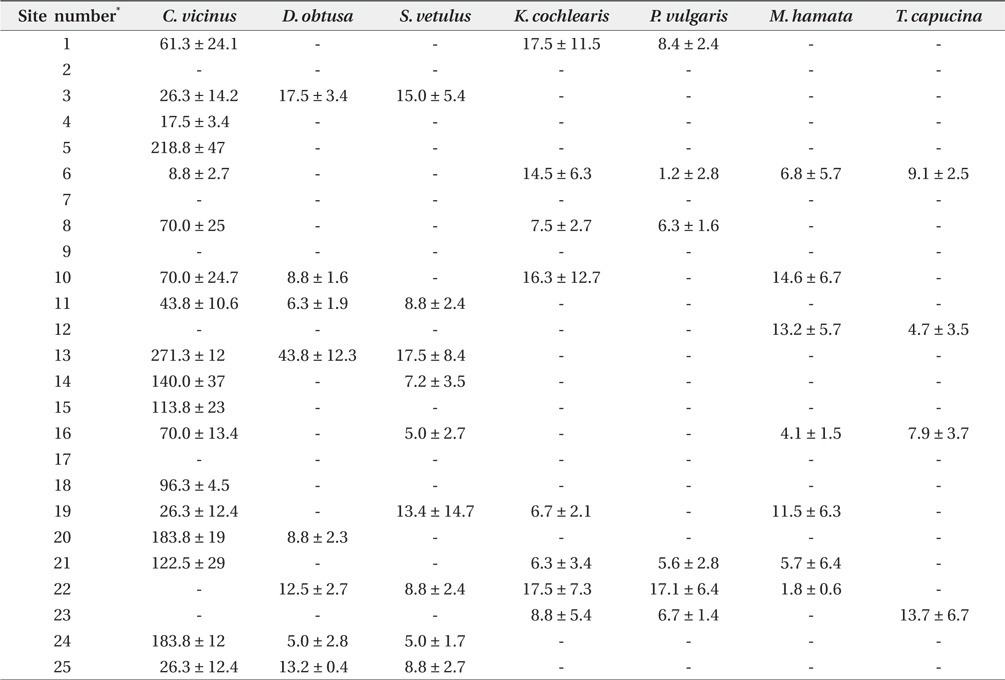
Zooplankton species density (individual/L) at study sites during winter
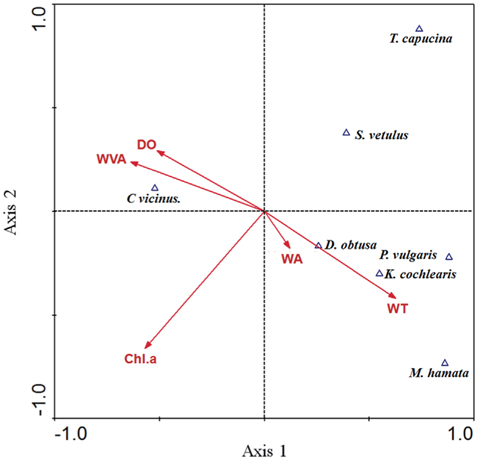
The results of CCA showed the relationship between environmental factors and zooplankton species (Fig. 2). The eigenvalues for CCA axis 1 and axis 2 were 0.73 and 0.46, respectively, and they accounted for 54.2% of the cumulative variance in the species data. The species–environment correlations for CCA axes 1 and 2 were high, and accounted for 68.2% of the variance in the species composition–environment relationship. Specifically, the withered vegetated area and DO had a strong effect on
In this study, we found high densities of
From results of the CCA analysis, we found that copepod density was related to withered vegetation area. Even though area of withered vegetation does not always be proportional to the occupied space by the vegetation, a strong positive relationship between
Non-copepod zooplankton species were partly analyzed by environmental parameters in the CCA. The presence of
In the present study, we hypothesized two possibilities for copepod (i.e.,
We expect that withered vegetation in water body supports tolerance of