Coastal sand dunes are formed by the movement of sand from the sea to land. At this time, the energy enforcing sand movement is derived from waves and wind. The physical environment for plant growth in coastal sand dunes is different from terrestrial ones; sand soil has good soil aeration but poor holding capacity of water and inorganic nutrients. In particular, coastal sand dunes shows a high soil surface temperature and low water content in summer, which is an unstable surface, as well as inadequate inorganic nutrients for plant growth. A small amount of salt is inputted continuously from sea water but it is easily leached by rainfall and the levels of nitrogen or phosphorus are inadequate (Chapman 1964, Rozema et al. 1985). In addition, soil nitrogen content is very low and it is main limiting factor to plant growth (Kachi and Hirose 1983, Berendse 1990, Olff 1992, Olff et al. 1993). For these reasons, plant species that can grow in coastal sand dunes are specific and limited.
Phytolacca americana is an exotic foreign species that was introduced into Korea in the 1950s and spread rapidly into the diverse vegetation after 1990 (Park 1995). This species was originally thought to acidify the soil, but this was later proven to be false (Park et al. 1999). Because of its adverse effects on the ecosystem, the Ministry of Environments of Korea designated P. americana to be a harmful species. Phytolacca americana tolerates strong acidic soil better than other species, and prefers strong sunlight. Moreover, its germination rate is high under sunny conditions (Joo and Cho 1995, Park et al. 1997, 1998). Park et al. (1998) reported that the growth of P. americana is dependent more on the sunlight than on the inorganic nutrient condition, and decreases significantly under 8% of the full sunlight, regardless of the inorganic nutrient content. Leaf area growth of P. americana increases conspicuously and the lowered net assimilation rate is compensated for by the increased leaf area under 33% full sunlight. The specific leaf weight is depended on the irradiation amount on the leaves. Joo and Cho (1995) reported that P. americana preferred intense sunlight. However, the small amount of inorganic nutrients acted as limiting factors for P. americana to grow in coastal sand dunes. Based on the fact that P. americana is commonly observed under a Robinia pseudoacacia canopy, inorganic nutrients, particularly nitrogen, as well as sunlight are believed to be important factors for the growth of this species (Joo and Cho 1995). Robinia pseudoacacia belongs to the Leguminosae family and supplies fixed nitrogen to the soil. As a result, these two species can be distributed together in the same area where the nutrient level is insufficient (Joo and Cho 1995).
In Korea, P. americana has threatened other peripheral plants as well as P. esculenta, which is a similar species, but is used as a pharmacological material. Therefore, many studies of P. americana have been carried out. These studies include its pharmacological effect and chemical composition (Woo and Kang 1975), main environmental factors controlling its growth (Joo and Cho 1995,Ri and Chun 1996, Park et al. 1998, 1999a, Park 2003, Kim et al. 2008), allelopathic activity (Bae et al. 1997, Kim et al. 2005a, 2005b), seed germination properties (Kang et al. 1997), effects on soil acidification (Kim 1996, Jang and Kim 1996, Park et al. 1999b). However, although P. americana has extended its distribution rapidly and was designated a harmful species by the Korean government, there is insufficient ecological information on the natural population of this species. As other coastal sand dune, Sindu sand dune is poor in soil nutrients but P. americana which prefers much nitrogen in soil is distributed in high density in a some area.
The aim of this study was to determine the main factors on the spatial distribution of P. americana in coastal sand dunes. For this, the sediment properties as a physical environment, vegetation as a biotic factor, and the age composition of P. americana at the same area at a population level were analyzed in the Sindu sand dune (Sindusagu), where the Cultural Heritage Administration of Korea has reserved it as a natural monument.
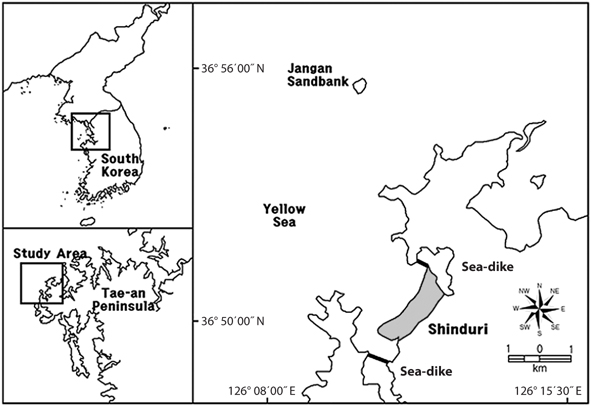
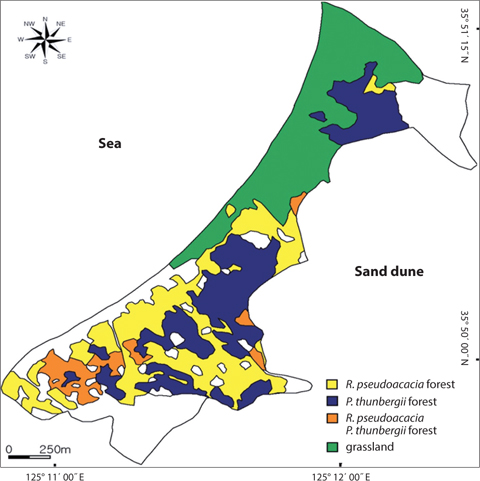
The study area was located at Sindu sand dune, Sindu- ri, Wonbuk-myeon, Taean-gun, Chungnam Province (36°50′ N, 126°16′ E) (Fig. 1). The Sindu sand dune is 2 km in length and 500 m in width. North-East area of Sindu sand dune is reservation area designated Natural Monument No. 431 by Cultural Heritage Administration of Korea (Taean-gun 2004). Previously, only the sand dunes of the South-Western small area were used as cultivated land and a residential quarter. However, with the exception of the reservation area, almost all areas on the seaside and forest have been seriously disturbed by the increase automobile roads. In particular, automobile roads are covered with gravel and soil from other areas, or are paved by cement. Therefore, studies of the sand dune vegetation have been carried out mainly in the reservation area (Ahn 2003, Song et al. 2005, Kahng 2006, Song and Cho 2007).
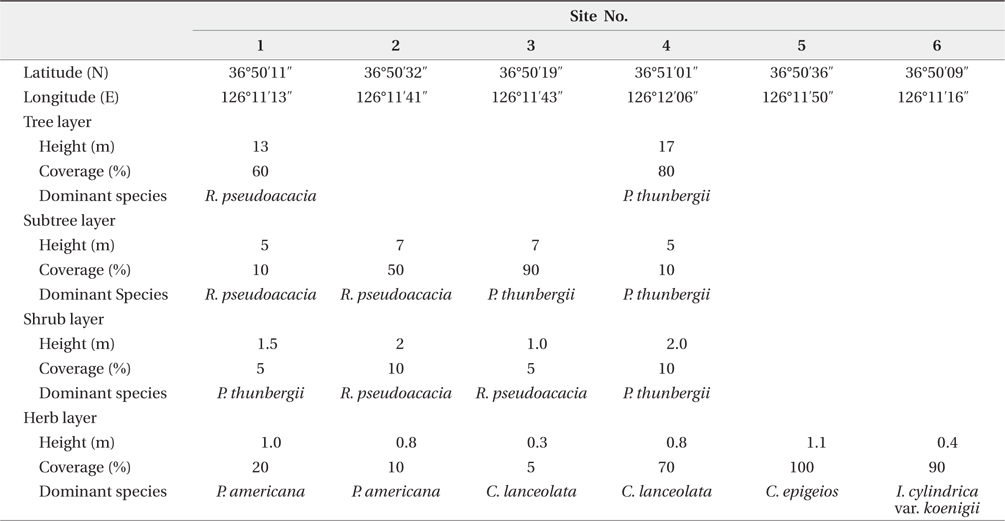
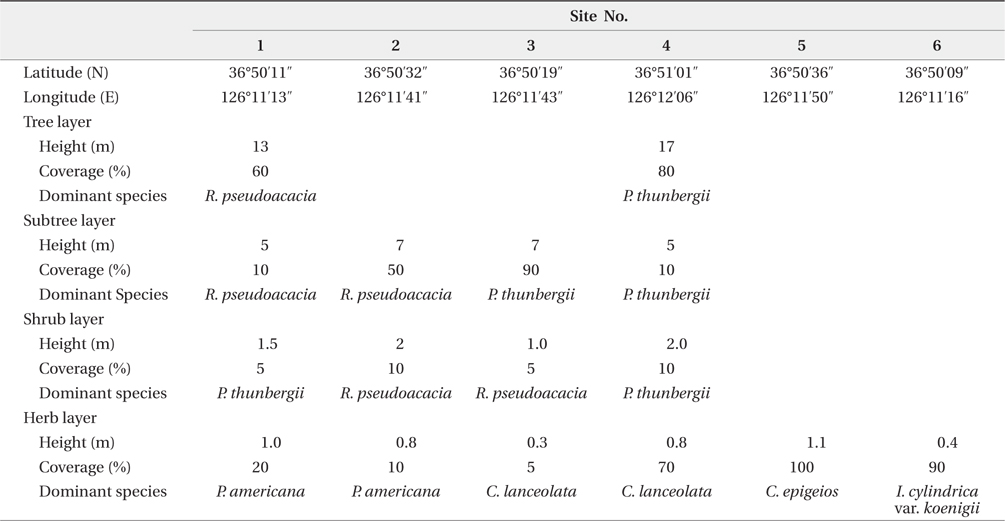
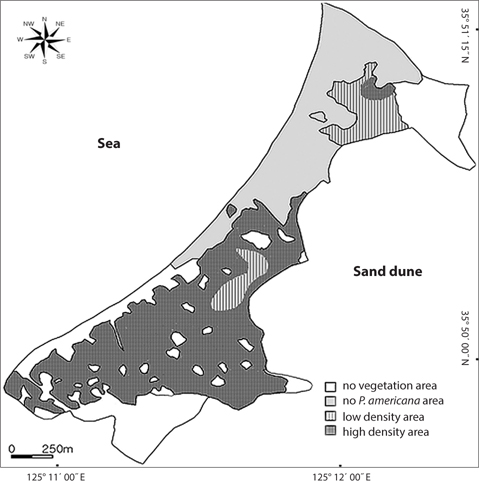
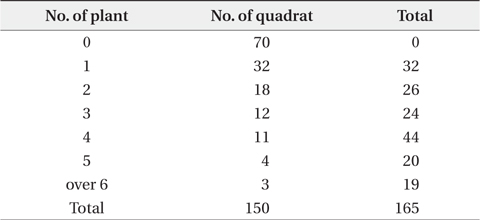
A field survey from June 2004 to February 2006 was accomplished in three ways. In 2004, the vegetation survey program for the coastal sand dune was carried out by Ministry of Environment. At this time, the distribution pattern of P. americana was outlined. Next, the relationship between fluting and plant size was surveyed in autumn of 2005. Nutrients of sediment were stable in winter, therefore, surveyed from November 2005 to February 2006. Firstly, to clarify the relationship between vegetation and P. americana, and spatial distribution of this species, the vegetation and density of P. americana in all areas was surveyed from June to August 2004. The vegetation was first grouped into the two communities: herbaceous (including Rosa rugosa and Vitex rotundifolia community) and woody communities. The latter was divided repeatedly into three groups: Pinus thunbergii, P. thunbergii-R. pseudoacacia and R. pseudoacacia communities. Of these, for the three communities (two woody and one herbaceous community), two representative sites were chosen and surveyed in 10 m × 10 m quadrat, respectively. Community structures were analyzed by Braun-Blanquet (1964). Woody community was classified into tree, subtree, shrub and herb layers. Next, height, dominant species and total coverage of each layer were checked. Coverage was grouped into 10% unit (5% unit in herb layer). The density of P. americana was checked by ordinary sight and in 10 m × 10 m quadrat. The data was divided into three categories of no, low density (under 0.1 plants/m2) and high density areas (over 0.1 plants/m2). To test the Poisson distribution of P. americana, 150 quadrats, 2 m × 2 m in size, were set up continuously along the 3 lines, 100 m in length. All shoots of P. americana were checked in a quadrat. Secondly, to clarify the relationship between R. pseudoacacia and P. americana, sixty four circular quadrats, 5 m in diameter, were randomly set up at woody community, and woody plants and P. americana were surveyed from November 2005 to February 2006. In the winter, there are two advantages in field sampling. Firstly, the decomposition rate of organic matter is low and the nutrient content leached from the sediment by precipitation is small, so that the sediment nutrient content is relatively constant for long time. Secondary, the biomass of herbaceous plant is limited in underground part and the exchange of organic matter between shoot and root is not, so that the datum for plant size is definite. At this time, the center of each quadrat was located on the largest R. pseudoacacia plant in a quadrat. The perimeters and ages of R. pseudoacacia and P. thunbergii were checked at 50 cm height from the sediment surface. The roots of P. americana were dug out, age-counted, dried at 80ºC in a dry oven and weighed. A sediment sample in a quadrat was collected at a 10 to 20 cm depth. The sediment samples were air-dried in the shade. The quadrats where stumps of P. thunbergii or R. pseudoacacia were present were omitted in statistical analysis of the relationship between P. americana and the former two species. Thirdly, to clarify the factor of the two, age and size, more affecting on flowering, the 230 plants which heights were in the range of 30-60 cm were sampled on September 2005. Sampled P. americana plants were grouped into fruiting plants (120 plants) and non-fruiting ones (110 plants). Age and root dry weight for all plants were checked. This survey could reveal that there was relationship between the age distribution of P. americana and the seed input at the same area or not.
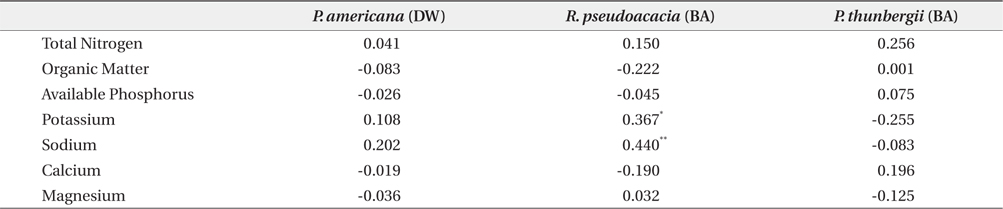
In sediment samples, the total nitrogen, organic matter and available phosphorus were analyzed using the micro-Kjeldahl method, loss on ignition method, and spectrophotometric method at 660 nm after molybdophosphoric blue coloring, respectively. The K, Na, Mg and Ca contents in the sediment samples were analyzed by inductively coupled plasma-atomic emission spectroscope (ICP) after water extraction. The spatial distribution of P. americana was analyzed using a Poisson distribution test. All correlation coefficients between the two factors were examined using the formula, y = ax + b.
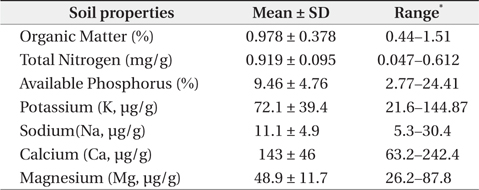
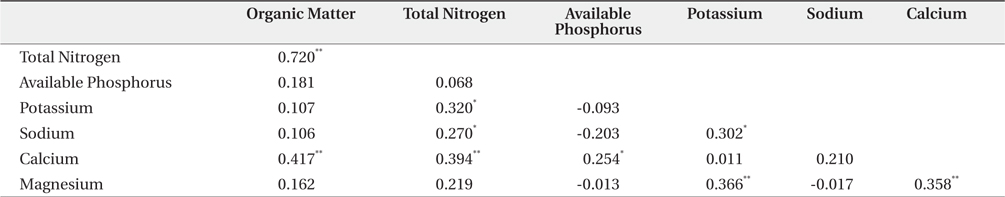
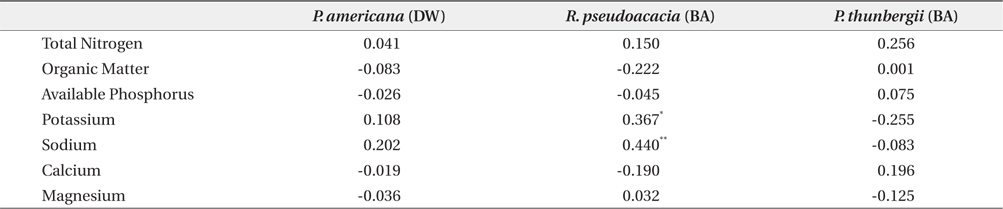
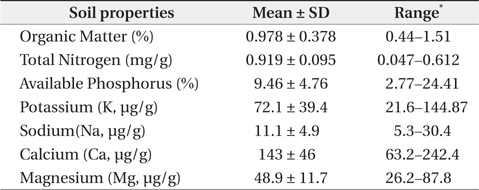
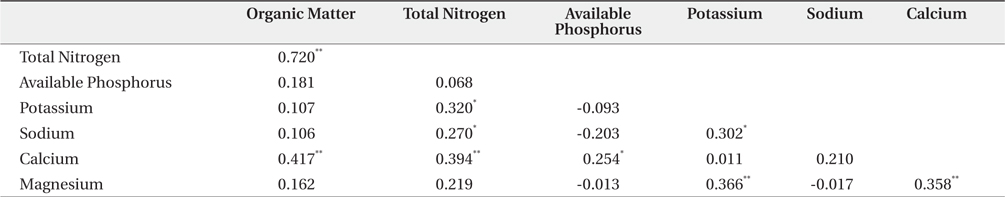
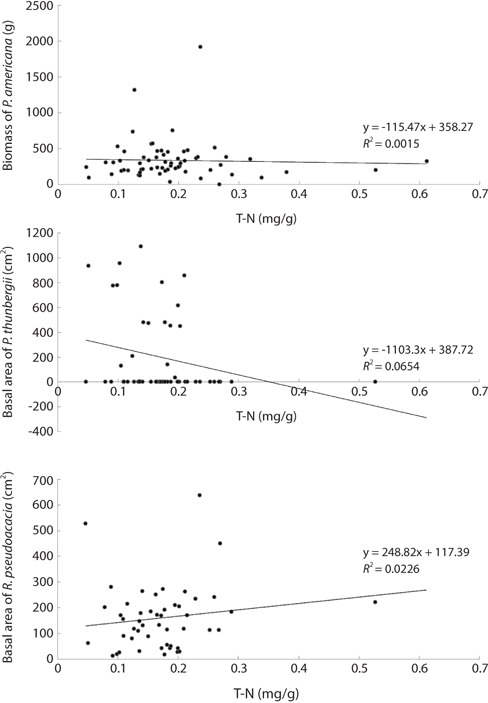
The mean and range (in sixty four quadrats) of sediment organic matter were 0.987 ± 0.378 and 0.44 to 1.51, respectively (Table 1). The total nitrogen content of sedimobile acment was 0.189 ± 0.095 mg/g ranging from 0.047 to 0.612 mg/g. The available phosphorus content in sediment was 9.46 ± 4.76 μg/g ranging from 2.77 to 24.41 μg/g. The sediment nutrient content ranged from 21.6 to 153.3 μg/g in K, 5.3 to 30.4 μg/g in Na, 63.2 to 287.0 μg/g in Ca and 26.6 to 80.4 μg/g in Mg. The nutrient content differed according to the sites. Five correlation coefficient (CC) values were significant at a 1% level (Table 2). The organic matter and total nitrogen, organic matter and Ca, total nitrogen and Ca, Ca and Mg, Mg and K showed a positive relationship. Four CCs were significant at a 5% level. Almost of the CCs between sediment properties and basal area or biomass of three plant species were not significant (Table 3). However, the CC between basal area of R. pseudoacacia and Na or K was significant at a 1% or 5% level.
The Sindu sand dune was composed of vegetation and non-vegetation areas. The non-vegetation area was road, pension, resident and cultivated land. The vegetation area was divided into three communities: P. thunbergii, P. thunbergii−R. pseudoacacia, R. pseudoacacia and mixed herbaceous communities (Fig. 2). The P. thunbergii community was formed in the broadest area except for herbaceous vegetation. The canopy heights were 7 m and 17 m in the two typical P. thunbergii stands (Table 4). Pinus thunbergii or R. pseudoacacia grew in the shrub layer but coverage of these species was <10%. In the P. thunbergii community, the coverage of R. pseudoacacia varied according to the location. Robinia pseudoacacia community distributed in small fragments along roadsides or cultivated areas. Coverage of the canopy layer was relatively low with a value of 50−60%. Robinia pseudoacacia was the dominant species in the subtree or shrub layer, and the coverage was <10%. The dominant species and coverage of the herb layer were P. americana and 10−20%, respectively. In the herb layer, the dominant species was Carex lanceolata in two sites but the coverage was different. The mixed herbaceous community was distributed in the largest area and was composed of a range of species. In the I. cylindrica var. koenigii community and C. epigeios community, the coverage was 100% and 90%, respectively, and heights of the dominant species were 110 cm and 40 cm, respectively.
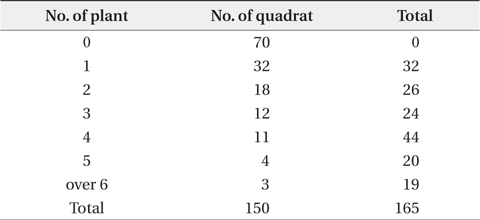
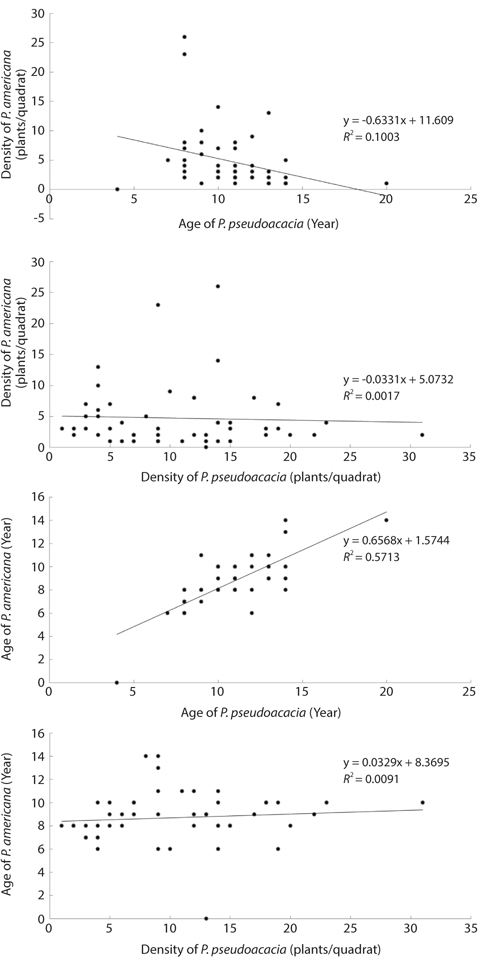
Distribution types of P. americana were divided into three groups according to the density (Fig. 3). As a result, firstly, the high density areas over 0.1 plants/m2 were all of the R. pseudoacacia community, the P. thunbergii-R. pseudoacacia community and a part of the P. thunbergii community. Secondly, the low density areas below the 0.1 plants/m2 were mainly the P. thunbergii community. There was virtually no P. americana in the P. thunbergii community bordering the herbaceous community. Thirdly, there were no P. americana areas in the herbaceous community. By the Poisson distribution analysis, dispersion pattern of P. americana was severely clumped and significant at a 0.001% level at the two communities - P. thunbergii−R. pseudoacacia community and R. pseudoacacia one (Table 5). The CC between the biomass of P. americana and basal area of R. pseudoacacia was significant at a 1% level (R2 = 0.138, n = 49) (Fig. 4). However, the CC between the biomass of P. americana and basal area of P. thunbergii was not. The ages of P. americana and R. pseudoacacia of the total area ranged from two to thirteen (mainly 3-11 years) and from three to twenty (mainly 4-15 years), respectively, except for one site. The CCs between the age and the density of two species (P. americana and R. pseudoacacia) were significant or not (Fig. 5). That was, the CC between the P. pseudoacacia’s age and P. americana’s one was significant at a 0.1% level (R2= 0.5713, n = 50), and its regression curve was y = 0.6568x + 1.5744. The CC between the age of P. pseudoacacia and the density of P. americana was significant at a 5% level (R2= 0.1003). However, the other two CCs were not significant.
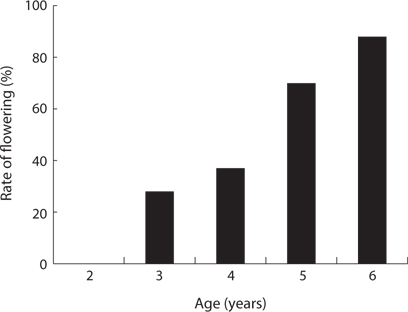
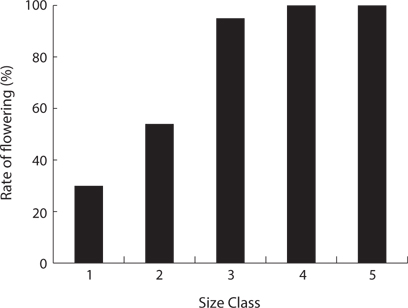
The CVs of a total area and a quadrat were 1.061 and 0.196, respectively (Table 6). The ages of P. americana in the total area were quite diverse but those in a quadrat (in 5 m diameter circle) were uniform. The other hands, only P. americana over 3 years old flowered and its rate generally increased with age (Fig.6). In particular, the flowering rates in a 3- and 6-year plant were 25% and 82%, respectively, so that plants of all age could flower, except for 2-year plants. Flowering rates were 28% at <2.0 g, 95% at 6 to 8 g, and 100% at >8.0 g (Fig. 7). The trend of the flowering rate was similar to age’s one. The difference between the smallest class and the largest one was more in the root dry weight than in age.
Many factors affected on spatial distribution of P. americana. Among these factors, sediment properties, vegetation and population properties were important. Firstly, in sediment nutrient content, this result was similar to that reported by Song et al. (2005), who surveyed the same area but only herbaceous vegetation area. That was, the organic matter content of this study area was lower than that 1.73% in the B layer, which was the lowest value in inland soil (Jeong et al. 2003). This showed that the organic matter content of coastal sand dunes was quite low, which was believed to be a common phenomenon (Olff et al. 1993). The total nitrogen content of the sediment differed considerably according to the sites. This result was similar to that (0.011% to 0.050%) reported by Song et al. (2005), but much lower than that (0.9 to 1.0 mg/g) in inland soil (Jeong et al. 2003). Especially, total nitrogen is limiting factors to growth of sand dune plants (Olff et al. 1993). The order of sediment nutrient content at this study area was Ca > K > Mg > Na, whereas that of sea water was Na > Mg > Ca > K. Therefore, it was believed that sea water did not affect the sediment nutrient composition directly or leaching property (mobility) of each nutrient in sediment differed. The CCs which were positive and significant at a 1% level showed the two series. One was the organic matter-total nitrogen-calcium and it was related to the organic substance group. The other was calciumpotassium- magnesium and inorganic substance group. The reason why sodium and available phosphorus were excluded was thought to be needed more studies.
Sediment nutrient contents were not related to plant sizes. Therefore, the relative biomass was not indicator of the sediment nutrient contents. This was attributed to the very low sediment nutrient content and the complex state among plants, rainfall and sediment. Generally, the nitrogen level in sediment increased with increasing R. pseudoacacia’s biomass. The lack of a relationship between the total sediment nitrogen content and the basal area of R. pseudoacacia was attributed to decomposition of the dead part of this species during the growth season and the leaching and reuse under the insufficient condition. The correlation coefficient between the P. americana’s root biomass and the basal area of R. pseudoacacia was significant at a 1% level but there was no correlation between the other two species. Therefore, it was thought that R. pseudoacacia affected on the growth and distribution of P. americana. Generally, soil nitrogen fixed by symbiotic bacteria with legumes affects the seed size and C/N ratio of P. americana (He et al. 2005), as well as shoot growth (Olff et al. 1993). Therefore, soil nitrogen is indispensable for the growth of P. americana. However, there was no positive relationship between the biomass of P. americana and the total nitrogen of sediment. To clarify this fact, more studies were needed.
Secondary, the vegetation of Sindu sand dune had been mainly studied at herbaceous area (Ahn 2003, Min 2004, 2006, Song et al. 2005, Kahng 2006, Song and Cho 2007), and by these reports, Elymus mollis, Carex kobomugi, Ischamum anthephoroides, Imperata cylindrica var. koenigii, Calamagrostis epigeios and Calystegia soldanella formed a pure stand in several areas. As mentioned above, the height and coverage of the canopy layer in woody communities were more than 7 m and 50%, respectively. However, the height of the dominant species in the herbaceous community was ≤1.1 m, as shown in this study and others (Song et al. 2005, Song and Cho 2007). Therefore, the irradiance that P. americana could receive was more in the herbaceous community than in the woody community. Park et al. (1998) reported that the growth of P. americana was affected by light rather than the nutrient environment. The growth of P. americana decreased conspicuously under 8% natural irradiance, even though there were sufficient nutrients. P. americana compensated for the low net assimilation rate by the increase in leaf area under the 33% natural irradiance. None the less, P. americana was no in herbaceous community, as shown in this study and other (Kahng 2006). The density of P. americana was high in the R. pseudoacacia community or P. thunbergii−R. pseudoacacia one. Therefore, factors rather than light that affected the growth of this species might explain why P. americana was absent in the herbaceous community. One of the several factors might be no seed dispersal to this area or unsuitable sediment environments. However, P. americana’s seed dispersal to the herbaceous community was possible because this community was in contact with the R. pseudoacacia community. Therefore, the environment of the herbaceous community was unsuitable for seed germination or growth of P. americana. In particular, the sediment nutrient contents of the study area were quite low as mentioned above. This sediment property was reported in other coastal sand dunes (Olff et al. 1993, Song et al. 2005). However, this study failed to clarify that sediment total nitrogen content affected on the distribution of P. americana, and R. pseudoacacia raised the sediment total nitrogen content, although in inland the former was reported to be the dominant species of the herb layer in areas where the latter was dead (Joo and Cho 1995). Moreover, the CC between the root biomass of P. americana and the basal area of R. pseudoacacia was significant at a 1% level and the ages of two species were correlated at a 0.1% level of significance. By this result, it was thought that R. pseudoacacia affected on distribution of P. americana in a certain way. The way that R. pseudoacacia affected the distribution of P. americana needed more studies. The fact that two species have something in common with invasion to the disturbed area might be a reason. Moreover, sediment nitrogen content was low at a time but could be continuously inputted from P. pseudoacacia’s litter. In relation to vegetation or succession, P. americana was reported to be a pioneer species of the early successional stage (Hyatt 1998). However, in this study, there was no P. americana in the herbal vegetation. Instead, P. americana was found only in the woody vegetation of the middle successional stage. Therefore, P. americana was not always a pioneer species of the early successional stage. In R. pseudoacacia and P. thunbergii- R.pseudoacacia communities, the dispersion pattern of P. americana was seriously clumped. In the temperate zone, most species appeared to be clumped. The reason for this was that the seeds or fruits tend to fall close to a parent (Barbour et al. 1980). The seed of P. americana is dispersed by bird or rodent feces (Orrock et al. 2003). A spike of P. americana is composed of 10-30 fruits, with each containing six seeds. However, a field survey showed that distance between two plants was >20 cm. Therefore, several plants in a site had germinated from the seeds in a feces pallet. In the time that a spike rolled with the wind, the fruit parted from the spike and was buried by sand in winter. In this time, only the seed properly buried in sand could germinate. In particular, the seeds on the soil surface could be easily eaten by herbivores and the germination rate of those at a 10-cm depth increased (Orrock and Damschen 2007). In coastal sand dunes, the movement of sand is important for P. americana seed’s burial, and this may be happened a fruit. Therefore, the fact that only one plant among the six seeds located in a fruit had survived requires more study. However, the diverse factors -light, physisoil water and nutrients- affected on seed germination. Especially, P. americana preferred abundant light.
Thirdly, only P. americana over 3 years old flowered and its rate generally increased with age. Considering these properties, seeds could be introduced yearly after three years at the site which a plant germinated at the first, so a seed bank could form in an area where P. americana was distributed. Regardless of this, the ages of P. americana in a quadrat (5.0 m-diameter circle) were similar. However, large plants did not have an allelopathic effect on seed germination (Kim et al. 2005a, 2005b). Moreover, the ages of P. americana ranged from two to seven years in a quadrat. Therefore, a large plant might suppress the young one rather than germination during growth from seedlings, so plants of a similar age remained in a small area unit. Accordingly, more study of P. americana in coastal sand dunes would be needed. The other hands, in relation to flowering and plant size or age, the difference between the smallest and largest classes was more in the root dry weight than in age. Therefore, plant size was related more to the flowering of P. americana than the plant age. Generally, many authors reported a strong positive correlation between the plant size and reproductive output (Klinkhamer et al. 1992). Of the two plant size factors, the flowering of field plants was positively correlated with the shoot size (Verburg et al. 1996, Min 2000). However, the flowering rate in other plant species was strongly related to the root system biomass (Fortanier 1973, Kawano 1975, Barkham 1980, Min 2003). Therefore, the main factor affecting flowering differed according to the plant species but flowering was related to the plant size rather than age. Hence, P. americana was similar to other plant species in the fact that plant size affected more than its age in flowering.