The genus Erythroglossum was established by Agardh (1898) based on the vegetative characteristics such as marginal ramifications and angular-rounded cell shape, and he transferred five species of Delesseria (e.g., D. schousboei J. Agardh, D. balearica J. J. Rodríguez, D. woodii J. Agardh, D. bipinnatifida Montagne, and D. californica J. Agardh) to the genus Erythroglossum. Later, Kylin (1924) designated E. schousboei (J. Agardh) J. Agardh as the lectotype species of Erythroglossum, and he established another genus Branchioglossum for E. woodii (J. Agardh) J. Agardh (Wynne 1983). Mikami (1979) reported that E. bipinnatifidum (Montagne) J. Agardh also has the same characteristics as Branchioglossum. Wynne (1983) agreed with Mikami and made the new combination, Branchioglossum bipinnatifidum (Montagne) M. J. Wynne. A total of 12 species of the genus Erythroglossum are currently accepted world-wide, including the original three species, which were first transferred from Delesseria to Erythroglossum by Agardh (1898) (Guiry and Guiry 2013).
Kylin (1924) divided the family Delesseriaceae into 11 groups, which were assigned to two subfamilies (e.g., Delesserioideae and Nitophylloideae) on the basis of vegetative and reproductive structures. He additionally assigned the genus Erythroglossum to his Phycodrys group of the subfamily Nitophylloideae. Wynne (2001) proposed 23 tribes under two subfamilies, and he placed the genus Erythroglossum within the tribe Phycodryeae (Lin et al. 2001a). Recently, Lin et al. (2001a) established the third subfamily as Phycodryoideae, based on molecular analysis including large subunit ribosomal DNA and rbcL sequence data. They assigned the four tribes Phycodryeae, Myriogrammeae, Schizoserideae, and Cryptopleureae to the subfamily Phycodryoideae: however, they did not test any species of Erythroglossum.
In the tribe Phycodryeae (Wynne 2001), there are two groups with different procarp development: 1) Phycodrys-type having one carpogonial branch with two sterile groups, and 2) Polyneura-type having two carpogonial branches with one sterile group. Five genera including Erythroglossum, Polyneura Kylin, Sorella Hollenberg, Sorellocolax Yoshida & Mikami, and Womersleya Papenfuss are known to have the Polyneura-type procarp (Yoshida and Mikami 1991, 1996, 1997, Maggs and Hommersand 1993, Kim and Nam 1994, Lin and Kraft 1996, Womersley 2003, Díaz-Tapia et al. 2009). Erythroglossum is distinguished from Sorella by tetrasporangial position (Hollenberg 1943, Yoshida and Mikami 1991), from Womersleya by the presence of a midrib or vein (Lin and Kraft 1996), and Polyneura by the presence of a midrib (Maggs and Hommersand 1993). The genus Sorellocolax is not comparable to Erythroglossum in terms of size or their host (Yoshida and Mikami 1996). In Erythroglossum, morphological features such as thallus habit, blade shape, structure and branching, and veins have been used for separating species (Maggs and Hommersand 1993, Yoshida and Mikami 1997, Díaz-Tapia et al. 2009). Recently, rbcL sequence data have been used for comparing the taxonomic position among the species of the family Delesseriaceae (Lin et al. 2001b, Lin and Nelson 2010, Kim and Kang 2011).
Three species of Erythroglossum, namely E. minimum Okamura, E. pinnatum Okamura, and E. latum Yoshida & Mikami, are currently known to occur in the northwestern Pacific region, and two of these species (E. minimum and E. pinnatum) are in the Korean coast (Guiry and Guiry 2013). The aims of this study are to compare the morphological features of the new species and other Erythroglossum species and to investigate the phylogenetic relationships in the tribe Phycodryeae based on the analysis of rbcL gene. To compare the molecular data, we collected two species (E. minimum and E. pinnatum) of Erythroglossum and Polyneura japonica from Korea and Japan. Unfortunately, we could not collect E. latum. This is the first study comparing molecular data within the genus Erythroglossum.
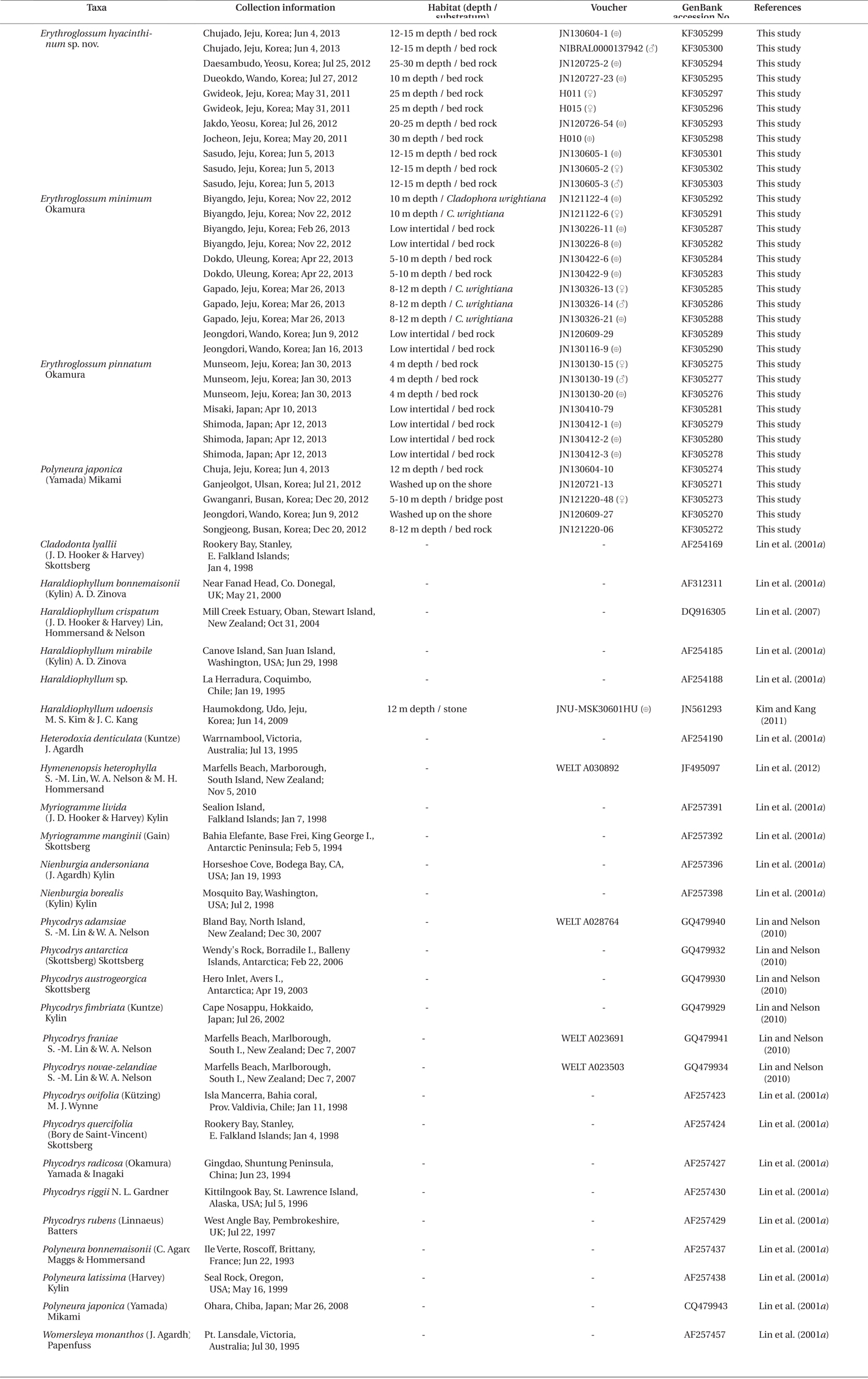
Specimens were collected from 15 localities of the Korean coast and two localities of the coast of Japan, from the intertidal and subtidal (Table1). Field-collected samples were put into an icebox with seawater and an icepack, and transported to the laboratory. The samples for DNA extraction were each assigned a voucher number, and a small part of the thallus was detached for making silica-gel dried tissue samples. The major part of the thallus was made into a pressed specimen and was deposited in the Herbarium of Jeju National University (JNUB), Jeju, and in the National Institute of Biological Resources (NIBR), Incheon, Korea. Samples for morphological observations were fixed in 5% formalin / seawater. Sections for microscopic examination were made by using a freezing microtome (NK-101-II; Nippon Optical Works Co., Ltd., Tokyo, Japan). For staining, 1% aqueous aniline blue acidified with a drop of 1% HCl was used. The stained sections were mounted in 30% Karo corn syrup. Photomicrographs were taken using a QImaging 1394 camera (QImaging, Surrey, BC, Canada) attached to a BX50 microscope (Olympus, Tokyo, Japan). For comparing molecular data, we used 25 Erythroglossum samples containing the new species from Korea, four E. pinnatum samples from Japan, five Polyneura samples from Korea, and 27 rbcL sequence data from the tribe Phycodryeae and Myriogrammeae (used as an outgroup) from previous studies.
For the extraction of total DNA from the silica-gel dried specimens, we used the same methods and primers as Kang and Kim (2013). The polymerase chain reaction (PCR) products were purified using the AccuPrep PCR Purification Kit (Bioneer, Daejeon, Korea) and were sequenced commercially (Macrogen, Seoul, Korea). Electropherogram outputs from each sample were edited using Chromas version 1.45 (McCarthy 1996). The total rbcL sequence was organized using the multiple-sequence editing program BioEdit (Hall 1999) and aligned visually (Kang and Kim 2013). None of the alignments posed a problem, as no gaps were observed.
Eleven rbcL sequences of the new species were aligned with 43 sequences of other species belonging to the tribe Phycodryeae, and seven species of Myriogrammeae were used as an outgroup. Maximum likelihood (ML) analyses were produced using RAxML (Stamatakis 2006) with the GTR + Г evolutionary model. We used 200 independent tree inferences by the -# option with default –I (automatically optimized SPR rearrangement) and –c (25 distinct rate categories) options of the program to identify the best tree. To generate bootstrap values we used the same program with the same settings for 1,000 replications (Kang and Kim 2013).
Description. Thalli erect, 3-6 cm high and 1.5-2.5 cm wide, lobed and membranous with a short cylindrical stipe, attached to the substratum by discoid holdfast, simple or divided di- or trichotomously; blades elliptical to obovate with rounded apex; blade margins beset with microscopic dentations; midribs conspicuous and faint near apical part of lobes, lateral veins divided from midrib alternately to dichotomously; microscopic veins absent; bright red with bulish-violet iridescence in living condition; cystocarps hemispherical with an ostiole, scattered over the monostromatic areas of the middle to upper blades; procarps consist of a supporting cell, a 7-8 celled sterile group, and two groups of 4-celled carpogonial branches; spermatangial sori produced on the lamellae between midrib and blade margin in the upper parts of blade; tetrasporangial sori round to irregular shape, scattered on monostromatic portions of middle to upper blades, composed of two layers of tetrasporangia; mature tetrasporangia spherical and divided tetrahedrally.
Holotype. JN130604-1 (tetrasporophyte), collected from 12 m depth of Chujado, Jeju province, Korea (33°58′04.28″ N, 126°17′08.64″ E) on Jun 4, 2013, and deposited in the Herbarium of Department of Biology, Jeju National University, Korea (JNUB).
Isotypes. JNUB (JN130604-3, JN130604-5 to -9) and KB (NIBRAL0000137942-3).
Etymology. The specific epithet (hyacinthinum) was chosen to represent the color of this species when alive, having a bulish-violet iridescence. Latin: hyacinthinus, -a, -um. adj. A.
Korean name. 푸른빛붉은혀
Habitat. Erythroglossum hyacinthinum was collected at 10-30 m depth where they were growing on bedrock.
Other specimens examined. Sasudo, Jeju on Jun 5, 2013 (JN130605-1, tetrasporophyte; JN130605-2, female gametophyte; JN130605-3, male gametophyte); Gwideok, Jeju on May 31, 2011 (H011 and H015, female gametophyte); Jocheon, Jeju on May 20, 2011 (H010, tetrasporophyte); Dueokdo, Wando on Jul 26, 2012 (JN120726-23, tetrasporophyte; JN120726-26, female gametophyte); Daesambudo, Yeosu on Jul 25, 2012 (JN120725-1, female gametophyte; JN120725-2, tetrasporophyte); Jakdo, Yeosu on Jul 25, 2012 (JN120726-54, tetrasporophyte); Geomundo, Yeosu on Jul 25, 2012 (JN120725-42, female gametophyte).
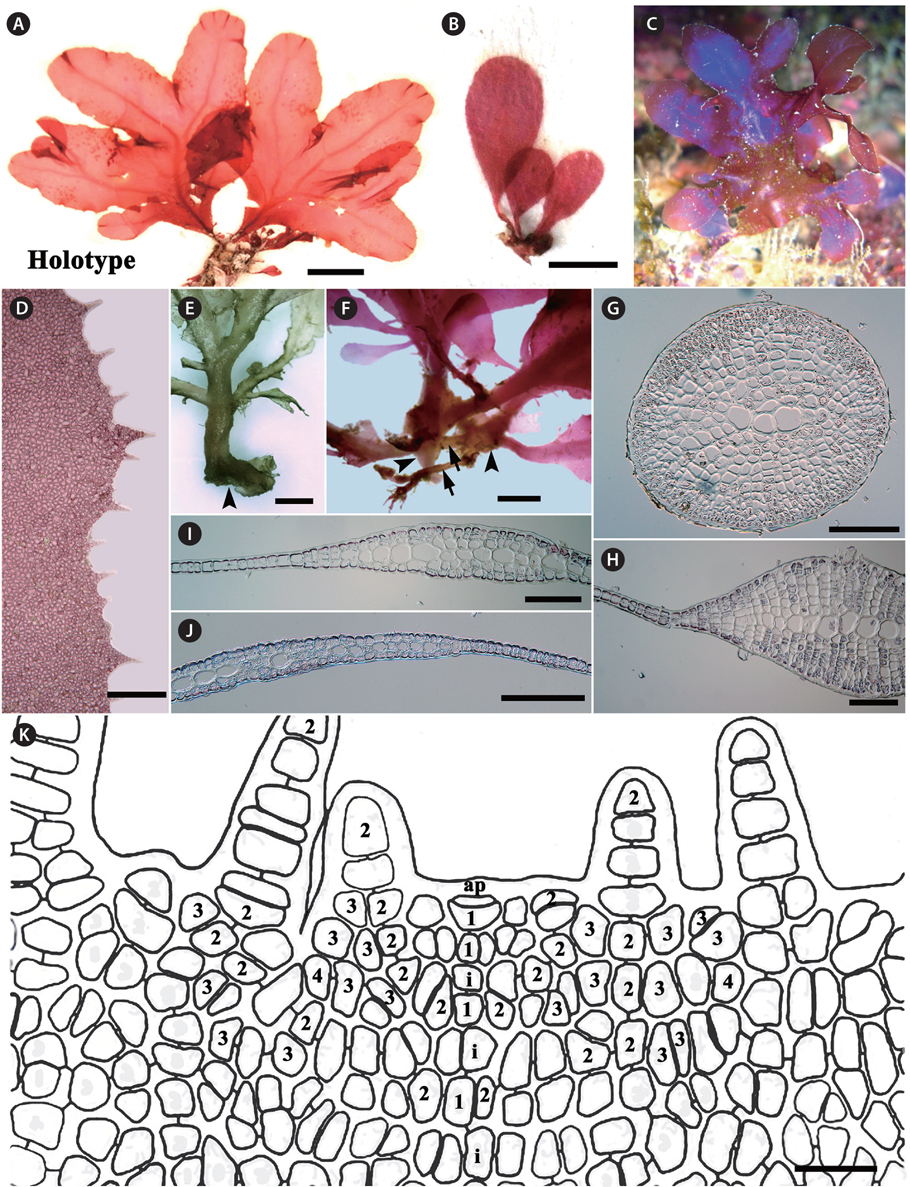
Morphology. Thalli are erect, broadly lobed, shortly stipitate, growing up to 3-6 cm high, 2.5 cm wide in the broadest portion of the lobes, and attached by a discoid holdfast (Fig. 1A-C & E). The blades are at first simple and elliptical to obovate in outline, and later divided ditrichotomously. Each lobe has an elliptical to obovate outline with a round apex (Fig. 1A-C). The margins of the blades are beset with microscopic dentations (Fig. 1D). The blades are mostly monostromatic, except for the midrib and lateral veins. The midrib is connected with a stipe, which is conspicuous except near the apical portion of the blade. Lateral veins divide alternately or dichotomously from the midrib (Fig. 1A, B, E & F). Microscopic veins or anastomosing nerves are absent. The basis of stipe produces creeping cylindrical branches, from which additional thalli are generated. The stipe is cylindrical in the basal part and is compressed near the starting point of the blade wing. Young blades irregularly initiate from the stipes (Fig. 1E-G). In the cross-section views, the veins are composed of large roundish cells, which are arranged side by side, and are located in the center of the branch with several cortical layers. The numbers of cortical cell layers decrease toward the apical part of veins (Fig. 1H-J). Fig. 1K shows the apical organization: the growth of thalli is initiated by an apical cell, which divides transversely generating the primary cell row. The cells produced by the transverse division of an apical cell are divided longitudinally to form second-order cell rows laterally. After producing second-order cell rows, certain cells of the primary cell row divide transversely, a process which is termed intercalary division, and also divide longitudinally. The second-cell rows reach the thallus margin, and some continue growth to develop microscopic dentations from the blade margin. The third-order cell rows are mostly cut off abaxially from the second-order cell rows, occasionally adaxially. Transverse and longitudinal intercalary cell divisions are frequent in the primary cell row and higher order cell rows.
[Fig. 1.] Erythroglossum hyacinthinum J. C. Kang and M. S. Kim sp. nov. (A) Holotype specimen from Chujado (JN130604-1), Jeju Island on Jun 4, 2013. (B) Habit of young thalli showing simple and elliptical to obovoid outline. (C) In the living condition, the thalli having bulish-violet iridescence. (D) Blade margin with microscopic dentations. (E & F) Basal part of thalli composed of discoid holdfasts (arrowheads) with creeping cylindrical branches (arrows) and short stipes. (G-J) Cross section views of stipe (G), the lower (H), the middle (I), and the upper part (J) of main branch: the blade composed of monostromatic lamellae and a polystromatic midrib. (K) Apical organization of thallus is showing primary (1) and higher order cell-rows (2-4), and cells resulting from intercalary divisions (i). Scale bars represent: A, 2 cm; B & D, 500 μm; E & F, 1,000 μm; G-J, 200 μm; K, 20 μm.
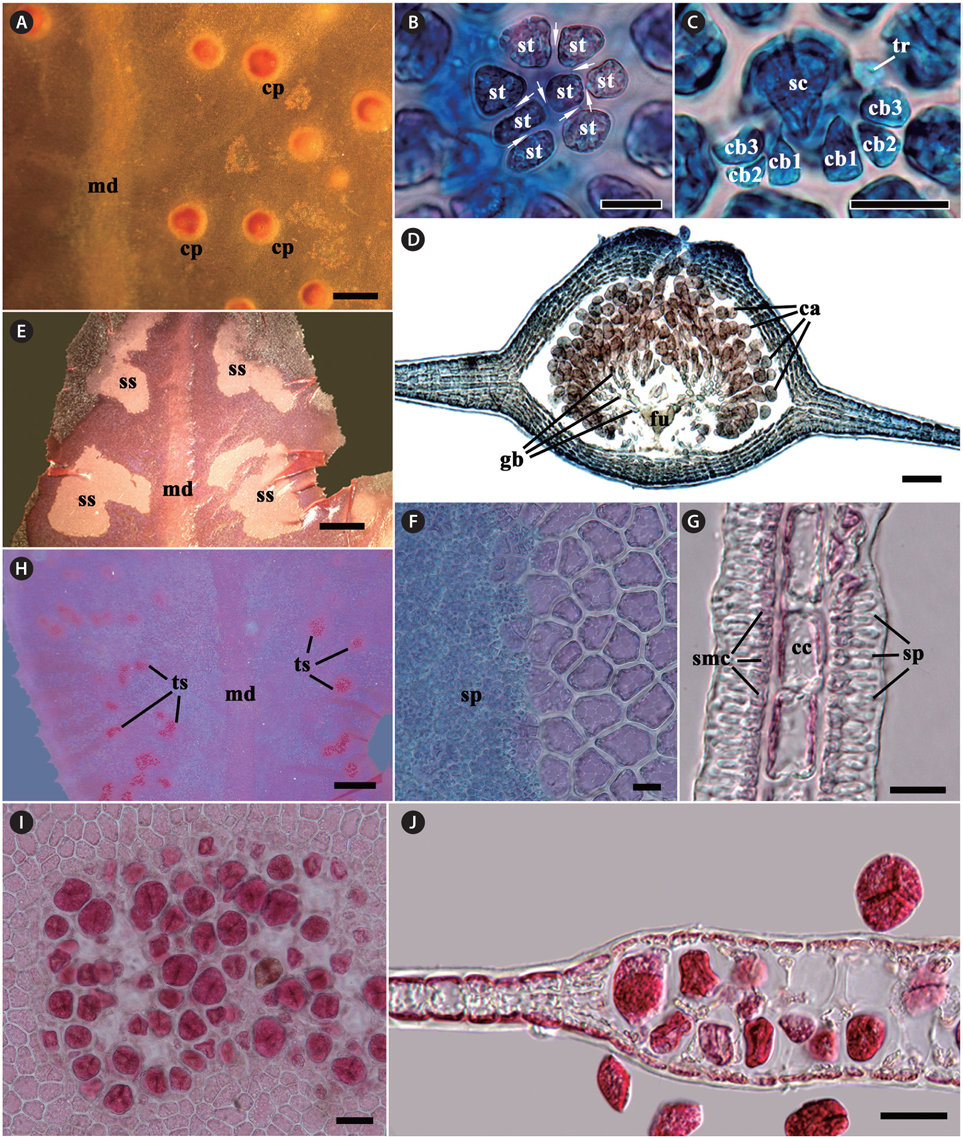
Gametophytes are dioecious. Procarps are scattered between the midrib and the blade margin in median to upper parts of blades, consisting of a supporting cell, a 7-8 celled sterile group, and two 4-celled carpogonial branches (Fig. 2A-C). Mature cystocarps are hemispherical and 500-700 μm in diameter, more swollen on the ostiolate side of blade, and composed of a large branched Hetfusion cell generating numerous radiate gonimoblast filaments, 2-4 chains of ovoid to pyriform carposporangia terminating each gonimoblast filament, and 5-7 cells thick pericarp (Fig. 2D). Spermatangial sori are produced between the midrib and blade margin in the upper parts of the blades, roundish to elliptical at first and later expand and become irregular in shape, consisting of a layer of central cells, two layers (each layer on opposite sides of central cells) of quadrangular spermatangial mother cells, which bearing elongate spermatangia (Fig. 2H-G). Tetrasporangial sori are scattered between veins and blade margin in median to upper parts of blades, circular at first and adjacent sori becoming coalescent as they expand, consisting of two layers of tetrasporangia. Mature tetrasporangia are spherical, 400-500 μm in diameter and divide tetrahedrally (Fig. 2I & J).
[Fig. 2.] Erythroglossum hyacinthinum J. C. Kang and M. S. Kim sp. nov. (A) Cystocarps (cp) scattered on the monostromatic lamellae between midrib (md) and blade margin. (B & C) Procarp composed of a supporting cell (sc), two groups of four-celled carpogonial branches (cb) with a trichogyne (tr), and a group of sterile cells (st), which are connected by pit-connections (arrows). (D) A mature cystocarp composed of a large branched fusion cell (fu), short-chained carposporangia (ca) on the terminal of gonimoblast filaments (gb), several cells layer pericarp, and an prominent ostiole on apical portion. (E) Spermatangial sori (ss) produced between midrib (md) and the blade margin. (F) Surface view of spermatangial sorus showing numerous spermatangia (sp). (G) Cross-section view of spermatangial sorus showing spermatangia (sp) produced by spermatangial mother cells (smc), which occur on opposite sides of central cells (cc). (H) Tetrasporangial sori (ts) produced between midrib (md) and blade margin. (I) Surface view of tetrasporangial sorus. (J) Cross-section view of tetrasporangial sorus showing two layers of tetrasporangia. Scale bars represent: A & H, 1,000 μm; B, C, F & G, 20 μm; D, 100 μm; E, 500 μm; I & J, 50 μm.
Molecular analysis. We determined a total of 61 rbcL sequence data: 25 Erythroglossum samples containing the new species from Korea, four E. pinnatum samples from Japan, five Polyneura samples from Korea, and 27 rbcL from the tribe Phycodryeae as putative relatives, and the tribe Myriogrammeae as an outgroup. We aligned 1187 nucleotide base pairs of rbcL gene, and of all sites, 383 (32.2%) were variable and 270 (22.7%) were phylogenetically informative. Eleven specimens of E. hyacinthinum from seven sites in Korea formed a clade with 0-0.2% divergence within the clade. E. hyacinthinum showed 2.7- 3.3% divergence from other Erythroglossum species, and 1.8-2.0% divergence from Polyneura japonica.
In the phylogenetic tree (Fig. 3), the clade containing Erythroglossum spp., P. japonica, and P. latissima was clearly separated from the other species of the tribe Phycodryeae, and strongly supported monophyly by the 100% ML bootstrap value. The clade of E. hyacinthinum formed a sister clade with P. japonica in a 70% bootstrap value. However, the Polyneura-type procarpic species (i.e., Erythroglossum spp., P. japonica, P. latissima, P. bonnemaisonii, and Womersleya monanthos) were not monophyletic. In contrast, the Phycodrys-type procarpic species (i.e., Phycodrys, Nienburgia, Heterodoxia, and Hymenenopsis) were monophyletic with low bootstrap support (69%).
Our data clearly indicates that we have collected a new species of Erythroglossum in Korea, E. hyacinthinum. This species does not match any other species presently described. In the tribe Phycodryeae, the five genera Erythroglossum, Polyneura, Sorella, Sorellocolax, and Womersleya are known to have procarp consisting of a pair of carpogonial branches and one sterile-cell group (Lin and Kraft 1996, Yoshida and Mikami 1996). The diagnostic morphological characteristics among the five genera are as follows: 1) the presence of midrib or midveins, and the marginal position of tetrasporangial sori for Erythroglossum; 2) presence of macroscopic midveins, and median position of tetrasporangial sori for Sorella; 3) midrib absent, presence of macroscopic and microscopic anastomosing nerves, and inter nerves position of tetrasporangial sori for Polyneura; 4) lack of microscopic and macroscopic veins, and growth by transversely dividing marginal apical cells, with the later formation of a continuous marginal meristem for Womersleya; 5) very small stellate and hemiparasitic thalli on the genus Sorella for Sorellocolax (Hollenberg 1943, Maggs and Hommersand 1993, Yoshida and Mikami 1996, Womersley 2003). The diagnostic characters of the genus Erythroglossum are entirely satisfied by morphology of our new species.
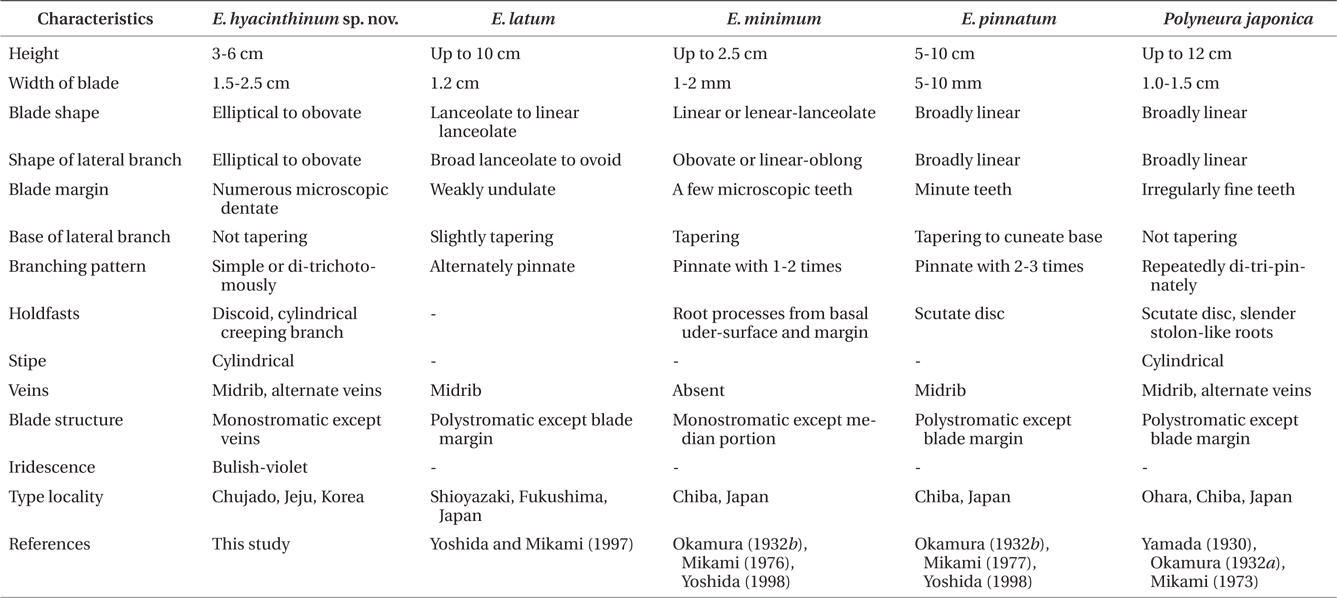
In the genus Erythroglossum, the following diagnostic characters for identification at the species level have been used: habit of thallus, branching patterns, and veins and blade structures (Maggs and Hommersand 1993, Yoshida and Mikami 1997, Díaz-Tapia et al. 2009). In observations of the morphological features, E. hyacinthinum is clearly separated from other species of Erythroglossum from the northwestern Pacific Ocean (Table 1): in terms of thallus size from E. minimum (3-6 cm high and 1.5-2.5 cm width vs. 1-2.5 cm high and 1-2 mm width), branching patterns from E. pinnatum (di-trichotomous vs. 2-3 times pinnate manner), and blade structures from E. latum (mostly monostromatic vs. mostly polystromatic). This new species more closely resembles Polyneura japonica rather than other Erythroglossum species in terms of discoid holdfast, cylindrical stipe, di-trichotomous branching, presence of midrib and lateral veins, and blade margins with fine teeth, but differs in blade structure (mostly monostromatic vs. mostly polystromatic), iridescence (bulish-violet vs. none), and shapes (elliptical to obovate vs. broadly linear) (Table 2). Upon first observation of E. hyacinthinum at 30 m depth, we were very confused as to its taxonomic status due to the extensive morphological similarities with P. japonica. However, we assigned the new species to the genus Erythroglossum because the morphological features (i.e., presence of midrib and lateral veins, marginal position of tetrasporangial sori) are more in agreement with the genus Erythroglossum than Polyneura (Maggs and Hommersand 1993).
P. japonica was originally described by Yamada as Hetfusion eronema japonica in 1930, and was then transferred to the genus Nienburgia in 1935 by Kylin (Yamada 1930, Mikami 1973). Later, Mikami (1973) confirmed that this species had Phycodrys-type apical organization and Polyneura-type procarp. At that time, Mikami was aware that the specimens had both midrib and lateral veins; however, he transferred the species to the genus Polyneura citing the presence of a midrib in Polyneura gmelinii (J. V. Lamouroux) Kylin. In Korea, Kim and Nam (1994) mentioned the possibility of establishing a new taxonomic group for P. japonica and P. gmelinii because these species have a midrib, lateral veins, and distinctive apical organization differently other species of Polyneura. However, P. gmelinii is currently treated as a taxonomic synonym of Erythroglossum laciniatum (Lightfoot) C. A. Maggs & Hommersand (Maggs and Hommersand 1993). The results of our molecular analyses demonstrate the possibility that P. japonica is more closely related with the genus Erythroglossum than Polyneura (Fig. 3). To fully resolve this issue, we need more morphological and molecular evidence from other taxonomic groups, including the type species of each genus, Erythroglossum, Polyneura, and Sorella.
In conclusion, we collected a new species that has similar morphological features with P. japonica. To confirm the taxonomic position of that species, we collected other species of the tribe Phycodryeae and performed morphological and molecular analyses. As the result, we identified a new species, E. hyacinthinum sp. nov. In addition, our molecular phylogenetic results highlight the problems of taxonomic position in the genera of the tribe Phycodryeae, namely showing the polyphyletic taxon among Polyneura-type procarp group.