Electrochemical double layer capacitors (EDLCs) are energy storage systems which can be used in portable electronic devices, hybrid vehicles and for storing the energy generated by solar cells. The electrical charge is stored in the electrochemical double layer formed at the interface between electrode and electrolyte [1-6].
Commercial EDLCs have activated carbon as active materials and quaternary ammonium salts in acetonitrile or propylene carbonate as solvent. Generally, these EDLCs have operating voltages of 2.7-2.8 V [7,8]. Activated carbons are the most generally used electrode materials, due to their great adsorption ability and high surface area compared to other materials [9]. Another factor that influences the characteristics of an EDLC is the electrolyte component. Usually, the organic solvent used as the electrolyte must have a specific dielectric constant over 20, which influences ion association. Since ethylene carbonate (EC) solvent has a high dielectric constant (εr = 89.6), it can effectively solve the salt. However, EC always has to be used with other solvents to drop its high melting point (Mp = 39°C). In contrast, linear carbonates such as 1,2-dimethoxyethane (DME), and dimethyl carbonate have low melting points and viscosity [10].
Many studies have been reported which aim to improve the electrochemical performance of EDLC by employing ionic liquids (ILs) [5,11-15]. ILs have an imidazolium, pyrrolidinium or solfonium based cation and an anion such as tetrafluoroborate (BF4 - ) or bis(trifluoromethansulfonyl)imide (TFSI-). The properties of ILs include non-flammability, wide voltage window and high ionic conductivity. The most common IL is 1-ethyl-3methyl imidazolium tetrafluoroborate (EMImBF4), which has a relatively low viscosity and high conductivity [16-18]. However, aromatic quaternary ammonium cations including imidazolium and pyridinium have relatively low cationic stability compared to pyrrolidinium [19].
Recently, it’s been suggested that PYR14BF4 may display good electrochemical performance and could be an interesting additive candidate for EDLC. This study is to evaluate the effect of PYR14BF4 additive on the optimal proportion of EC/DME mixtures for application to EDLC.
2.1. Carbon electrode and electrolyte
The carbon electrodes were prepared using activated carbons (MSP-20) as the active materials, carbon black as conducting agent and carboxymethyl cellulose/styrene-butadiene rubber as binder, in a glove box with Ar atmosphere. The materials were mixed in a fixed mass ratio and coated on nickel foam substrate. The composition was dried at 100°C in a vacuum oven for 12 h. The average active materials mass loading was about 10 mg/cm2 .
EC and DME were purchased from DAEJUNG (>99%) and JUNSEI (>99%) respectively. Tetraethylammonium tetrafluoroborate (TEABF4) salt was purchased from Aldrich Sigma and N-butyl-N-methylpyrrolidinium tetrafluoroborate (PYR14BF4) was supplied by C-tri Co. Ltd. The electrolytes were prepared by dissolving different weight ratios of PYR14BF4 into the EC/DME (1:1) co-solvent with 0.1 M TEABF4 in a glove box. Then the prepared solutions were stirred for 24 h.
2.2. Measurement & analyse the characterization of EDLC
The ionic conductivity of the electrolytes mixture was evaluated by conductivity meter (ES-51, HORIBA Ltd.). Also, the bulk resistance and stability were evaluated by AC impedance spectroscopy over the frequency range from 100 kHz to 0.01 Hz and linear sweep voltammetry. In order to measure the operative voltages and capacitances of the EDLCs, cyclic voltammetry (CV) was carried out at 5 mVs-1 in three electrode systems. In this study, the working electrode was the prepared carbon electrode. Pt wire and Ag/AgCl (3 M NaCl) served as counter and reference electrodes, respectively.
3.1. Conducting property of electrolyte
Although EC exhibits high dielectric constant and viscosity, it always has to be used with another solvent due to its high melting point. So, for this purpose we selected 1,2-DME, a linear carbonate that has a low melting point (Mp = -58°C). The optimal mixture ratio is 1:1 vol%. The incorporation of a co-solvent should result in the reduction of the melting point because of dipolar interaction [20].
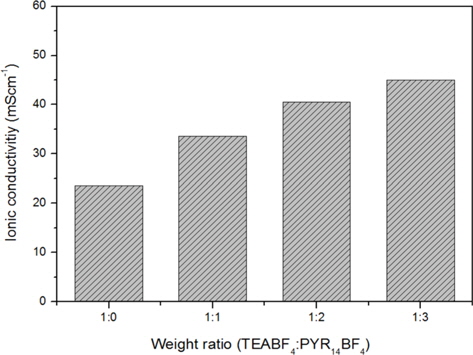
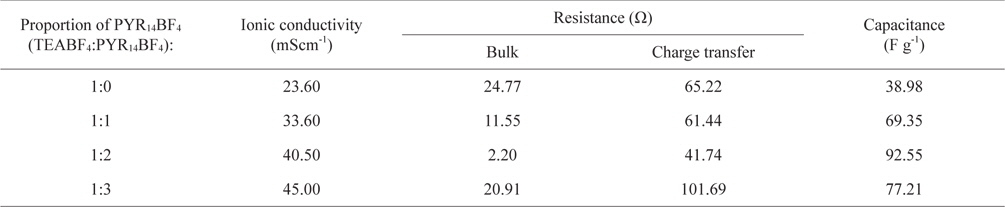
Fig. 1 indicates that the ionic conductivities of the electrolyte at room temperature increased gradually with added amounts of PYR14BF4 additives, because it introduced numerous charge carriers into the electrolyte. The detailed values of conductivities are indicated in Table 1. When adding the TEABF4:PYR14BF4 over 1:2, the ionic conductivity was not sharply changed due to the increase of ion carriers.
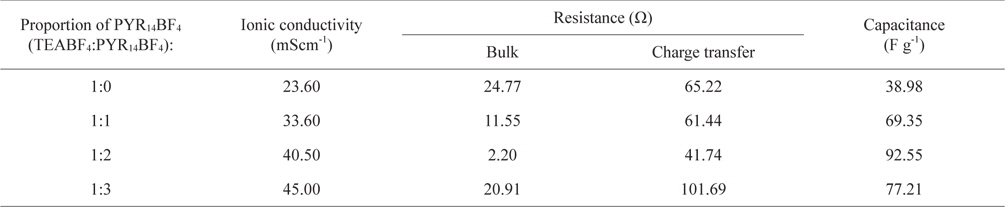
Ionic conductivity, resistance and capacitance for different proportions of PYR14BF4 in EC/DME (1:1) at room temperature
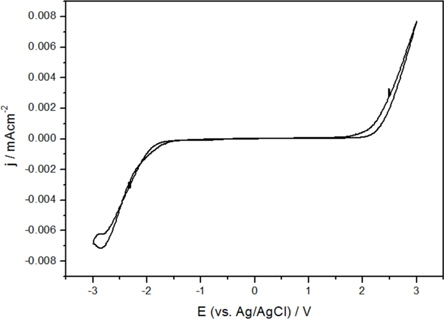
When preparing an electrolyte solution for a supercapacitor, electrochemical stability is an important factor. The stability of the electrolyte in this study was determined using CV at a scan rate of 10 mVs-1. Pt wires were used as the working and counter electrode and the Ag/AgCl electrode was used as the reference electrode. As shown in Fig. 2, the operative potential from -2 V to 2 V was electrochemically stable. The reason EC/DME with TEABF4:PYR14BF4 (1:2) was selected was because it has good electrochemical performance, such as capacitance, rate capability and resistance. In the electrolyte stability measurement, EC/DME with TEABF4:PYR14BF4 (1:2) was studied as an optimal electrolyte for a capacitor device.
3.2. Electrochemical performances of EDLCs
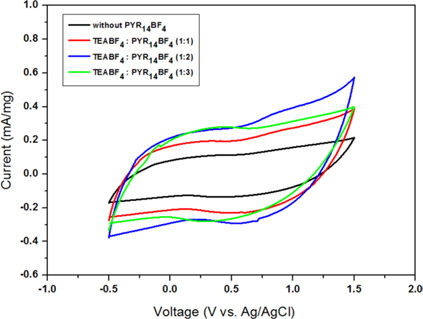
Fig. 3 shows the CV of EDLCs at a scan rate of 5 mVs-1. The CV curves exhibit rectangular shapes that are typical of the capacitive properties of an EDLC. The specific capacitance of electrodes can be calculated according to the following equation.
C (Fg-1) is the specific capacitance, I is the response current density (Acm-2 ), v is the scan rate of potential (Vs-1), m is the mass of the active materials in the working electrode (g), and ΔV is the operative voltage (V) in a three electrode system. The calculated capacitance values are indicated in Table 1.
As more PYR14BF4 ILs were added, the capacitances continuously increased, to 92.55 F/g. However, at the weight ratio of TEABF4:PYR14BF4 over 1:2, the capacitance decreased, and may have been saturated. From these results, its clear PYR14BF4 ILs have positive effects on the electrolyte itself and electrode.
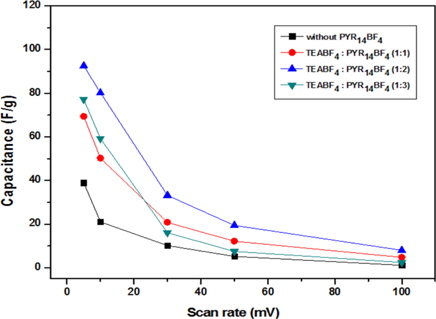
Fig. 4 displays the rate capability at different scan rates (5, 10, 30, 50 and 100 mVs-1) between -0.5 V and 1.5 V. It can be observed that specific capacitances decreased with increasing scan rates. The ions of the electrolytes had difficulty diffusing into the inner pores of the carbon electrode at high scan rates, so only the external surface area of the electrodes could participate in the ion transfer reaction. At a high scan rate of over 30 mVs-1 the capacitance using the TEABF4:PYR14BF4 over 1:2 declined sharply. This suggested that a number of ions obstructed the diffusion of ion charges. At the weight ratio of TEABF4:PYR14BF4 (1:2), good rate capability was confirmed, and the maximum specific capacitance was 92.55 F/g at 10 mVs-1 and 8.01 F/g at 100 mVs-1.
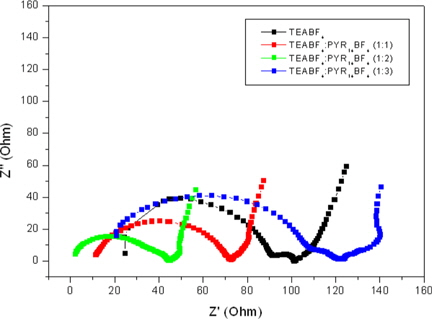
In Fig. 5, the impedance plots of co-solvents as a function of PYR14BF4 contents are displayed. The beginning plots at high frequency mean bulk resistances, and the size of the semi-circle indicates charge transfer resistance. The calculated resistance values are shown in Table 1. When the PYR14BF4 additives were added to the electrolyte, the bulk resistance declined and smaller sized semi-circle was observed. However, the bulk resistance and the charge-transfer resistance increased at a weight ratio of TEABF4:PYR14BF4 over 1:2. The charge transfer resistance is associated with gradients of potential between the ions in the electrolytes and the surface of the electrode. This phenomenon was caused by the kinetics of the electrochemical reactions and the diffusion of ions at the surface of the carbon electrode.
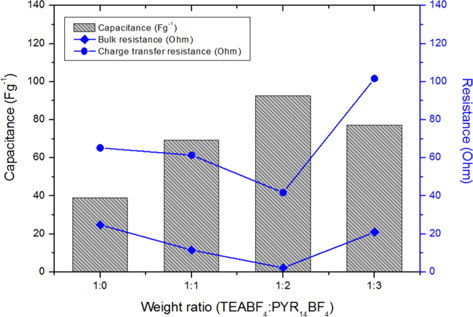
The capacitances have an effect on the charge transfer resistance [21,22]. Fig. 6 shows the variation of capacitance and resistances of bulk and charge-transfer produced by adding the PYR14BF4 additives. As can be seen, PYR14BF4 ILs can enhance the mobility of ion charge transfer and the capacitance of the carbon electrode. These results suggest that using the TEABF4:PYR14BF4 (1:2) in an organic electrolyte can produce an effective EDL and contribute to reducing the resistance at the carbon electrode, resulting in enhanced electrochemical performance.
In this paper, the effect of introducing PYR14BF4 additive into an electrolyte against a carbon electrode was investigated for use in a capacitor. The addition of PYR14BF4 into the organic solvent highly improved the ionic conductivity and reduced the interface resistance between the electrolyte and activated carbon electrode. Then, the PYR14BF4helped make a more compact EDL and so enhanced charge transfer throughout the activated carbon electrode. The maximum specific capacitance of the activated carbon electrode using the TEABF4:PYR14BF4 (1:2) additive in EC/DME (1:1) mixture electrolyte was 92.55 F g-1. The electrochemical performance of the EDLC containing the TEABF4:PYR14BF4 (1:2) in EC/DME (1:1) electrolyte can be further optimized by considering a similar concept of using specialized supporting salts having large volume sized cation and anion.