Nitrogen-doped microporous carbons were prepared using a polyvinylidene fluoride/melamine mixture. The electrochemical performance of the nitrogen-doped microporous carbons after being subjected to different carbonization conditions was investigated. The nitrogen to carbon ratio and specific surface area decreased with an increase in the carbonization temperature. However, the maximum specific capacitance of 208 F/g was obtained at a carbonization temperature of 800°C because it produced the highest microporosity.
Supercapacitors are used for energy storage in various portable electronic devices and hybrid electric vehicles. They possess higher power and energy density, longer cycle life, and higher chemical stability compared to secondary batteries [1-3]. Recently, various carbon-based electrodes have been employed in supercapacitors because of their good electrical conductivity and large pseudocapacitance; however, they exhibit low capacitance values [4-6]. To improve their capacitance, nitrogen-containing functional groups are incorporated into the carbon materials. The pseudocapacitance originates from the interaction between the nitrogen species and protons of the electrolyte [7-10].
Recent studies have revealed that mesopores and macropores in capacitor electrodes have limited capacitance [11,12]. The presence of narrow micropores is essential for the formation of an electrical double layer by solvated and desolvated ions. According to the literature, pore sizes ranging from 0.7-1.2 nm produce the optimum specific capacitance in aqueous electrolytes [13]. Therefore, producing microporous carbons with pore size in the abovementioned range is an effective way to improve the supercapacitor capacitance. Polyvinylidene fluoride (PVDF) is usually used as the polymer precursor for preparing the electrodes of supercapacitors. PVDF is converted to microporous carbon by carbonization without other activation methods. The microporous carbon has high specific surface area and potential. It also possesses narrow micropore distribution characteristics [14-17]. Melamine is a nitrogen rich compound, which is easy to handle. It is usually used for doping nitrogen functional groups. Nitrogen-enriched carbons possess high specific surface area and specific capacitance [18-21]. Nitrogen in melamine-derived carbons exists mainly in pyridinic, quaternary, and oxidized forms. The oxidized nitrogen is the most stable and the pyridinic nitrogen affects the electron donor-acceptor characteristics of the carbon materials, which leads to pseudocapacitive attraction between the protons of the electrolyte and the carbon electrode materials [22]. At a high carbonization temperature, more stable nitrogens are produced by the carbonization of melamine [23,24].
In this study, nitrogen-functionalized microporous carbons were prepared by an activation-free method using the PVDF/melamine mixture as the carbon precursor. The effect of carbonization temperature on the electrochemical performance of the PVDF/melamine-based electrode is discussed.
2.1. Materials and preparation
PVDF (Aldrich) and melamine (Aldrich) were used as the precursors for the nitrogen-functionalized microporous carbons. A mixture containing 1:0.5 ratio of the PVDF/melamine was put into a muffle furnace and was stabilized at 200°C in air for 2 h. Then, carbonization was carried out at 700-900°C for 2 h in a tubular furnace under nitrogen gas with a heating rate of 5°C/min and a nitrogen flow rate of 200 mL/min [6,25]. The samples were named PVDF/melamine 700, 800 and 900, where 700, 800, and 900 represent the carbonization temperature.
The surface characterization was performed by elemental analysis (EA). The textural characteristics of the PMs were analyzed at 77 K using a gas adsorption analyzer (BELSORP, BEL Japan). The specific surface areas and micropore volumes of the samples were determined from the Brunauer-Emmett-Teller (BET) and Dubinin-Radushkevich (D-R) equations. The electrochemical performance was characterized by cyclic voltammetry (CV) and galvanostatic charge/discharge measurements using a three-electrode electrochemical cell. The threeelectrode cell consisted of a Pt wire as the counter electrode, Ag/AgCl as the reference electrode, and Ni foam coated with the samples as the working electrode. The specific capacitances of the samples studied were estimated according to the following Eq. (1) [26,27]:
where
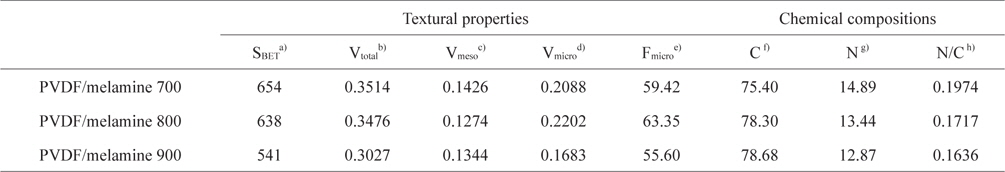
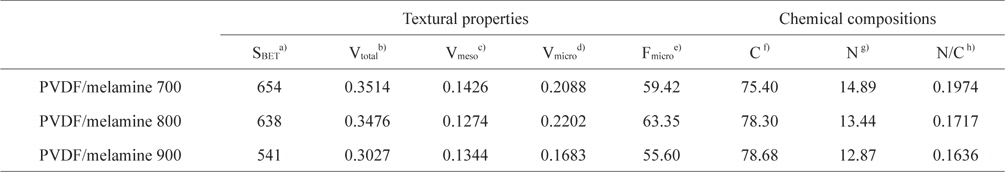
PVDF was used to obtain porous carbons by carbonization in the absence of an activation process. The specific surface area and pore volume of the samples studied were determined by nitrogen adsorption/desorption isotherms at 77 K. The textural properties determined by the BET results are shown in Table 1. The decrease in the specific surface area of the samples studied is attributed to the effect of carbonization temperature. The total pore volume also decreased. Therefore, the formation of the micropores could be easily controlled by the carbonization process. However, the highest micropore volume was obtained at 800°C. The PVDF/melamine 800 had the highest micropore volume (0.2202 cm3/g) and the lowest mesopore volume (0.1274 cm3/g), which is probably due to poor carbonization at the low temperature and the collapse of the micropores at the high temperature.
The surface characteristics of the samples studied were analyzed by EA. As shown in Table 1, the content of nitrogen groups on the PVDF/melamine 700, 800, and 900 are 14.9, 13.4, and 12.9%, respectively. The nitrogen content decreased with the increasing carbonization temperature. The PVDF/melamine 900 has the lowest nitrogen content (12.9%), which is probably due to the volatilization of the N/C species at elevated carbonization temperatures [31].
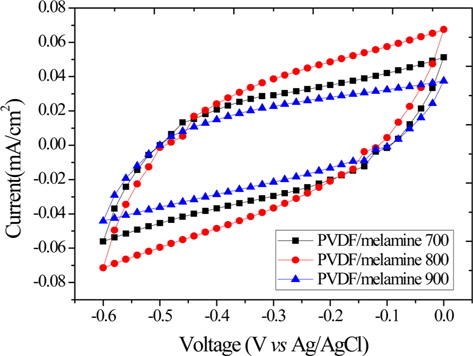
Fig. 1 shows the CV of the samples studied using 6.0 M potassium hydroxide electrolyte solution at the scan rate 50 mV/s in the voltage range of -0.6 V to 0.0 V. The CV curves are symmetric rectangular in shape and exhibit a larger peak area, which shows that the sample has a high specific capacitance. The largest CV curve obtained was for the PVDF/melamine 800, which shows that it has a greater current density than the other samples studied, resulting from the micropore volume (Table 1).
[Table 1.] Chemical compositions and textural properties of the samples studied
Chemical compositions and textural properties of the samples studied
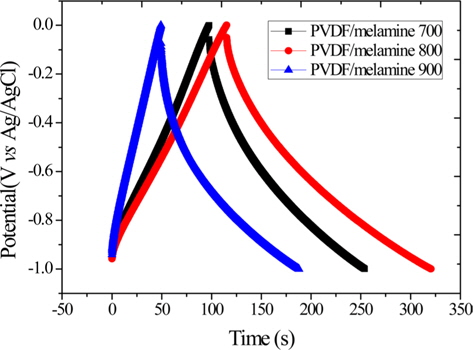
Fig. 2 shows the galvanostatic charge/discharge curve at a loading current of 1 A/g. The galvanostatic charge/discharge curve is linear triangular in shape. The curve for the PVDF/ melamine 800 shows the largest peak and the longest discharge time. The specific capacitance is calculated from the discharge curve (Fig. 2); and it indicates 156, 205 and 139 F/g on the PVDF/melamine 700, 800 and 900. The PVDF/melamine 800 exhibited the highest specific capacitance (205 F/g). The specific capacitances of the samples studied are dependent on the carbonization temperature, which in turn can control the pore size of the carbon and the contents of the polar doping groups. The increase in capacitance is attributed to the added micropores. The nitrogen-doped microporous carbon exhibited enhanced electrochemical performance when prepared at the carbonization temperature of 800°C, and demonstrated superior electrochemical supercapacitor and pseudocapacitive behaviors [32].
In this study, nitrogen-functionalized microporous carbons were prepared by carbonization of a PVDF/melamine mixture without an activation process. The nitrogen contents decreased with increasing carbonization temperatures. However, the micropore volume of the PVDF/melamine 800 was greater than that of the PVDF/melamine 700. The PVDF/melamine 800 sample had a maximum specific capacitance of 205 F/g with a large micropore volume of 0.2202 cm3/g. Thus, we found that the microporosity of the PVDF/melamine can be attributed to preparation at an optimum carbonization temperature, resulting in the easy transfer and excellent accessibility of the electrolyte ions in the nitrogen-functionalized carbon electrodes.