Due to their morphology, electrochemical stability, and function as a conducting carbon matrix, graphene nanosheets (GNS) have been studied for their potential roles in improving the performance of sulfur cathodes. In this study, a GNS/sulfur (GNS/S) composite was prepared using the infiltration method with organic solvent. The structure, morphology and crystallinity of the composites were examined using scanning electron microscopy, transmission electron microscopy, and X-ray diffraction. The electrochemical properties were also characterized using cyclic voltammetry (CV). The CV data revealed that the GNS/S composites exhibited enhanced specific-current density and ~10% higher capacity, in comparison with the S-containing, activated-carbon samples. The composite electrode also showed better cycling performance for multiple charge/discharge cycles. The improvement in the capacity and cycling stability of the GNS/S composite electrode is probably related to the fact that the graphene in the composite improves conductivity and that the graphene is well dispersed in the composites.
Lithium-ion batteries have been used as power source for many electronic devices. However, due to the increased demand from huge markets such as that for electronic devices, the cathode materials are now required to provide both high-capacity and environmentally friendly energy storage. For these reasons, energy storage systems become more important than ever before. One option for next-generation batteries involves lithium-sulfur batteries (LSBs), and they have recently attracted a lot of attention due to their high theoretical capacity (1675 mAh g−1) and high specific energy (2600 Wh kg−1) [1,2]. They also have the additional benefits of low cost and environmental compatibility. Despite all these advantages, LSBs have not yet been commercialize for two reasons. First is the dissolution of reactants (lithium polysulfides) into the electrolyte, and second is the low conductivity of elemental sulfur. It may be possible to develope a new electrolyte to prevent the polysulfides from dissolving, but there is no way to improve the conductivity of sulfur [3-5]. In response to these conditions, some strategies have been proposed to prevent the impact of the polysulfide shuttle effect. For instance, extensive research has been done to improve the electrical conductivity of the cathode and to trap the sulfur and polysulfides in a carbon matrix. For example, carbon matrices (i.e., porous carbons [6], carbon nanotubes [7], hollow carbon [8], carbon fibers [9], ordered mesoporous carbon [10], hierarchical porous carbon [11] and graphene nanosheets (GNS) [12] have been investigated to improve electrochemical performance by increasing the utilization of the sulfur cathode. Among these carbon materials, GNS can open new possibilities because of their high electrical conductivity, superior mechanical flexibility, high chemical and thermal stability, high surface functionality, and large surface area [13-19].
In this work, we prepared GNS/sulfur (GNS/S) and activated carbon/sulfur (AC/S) composites with a variety of sulfur content. In general, GNS has higher conductivity than AC composites. With this in mind, we synthesized a combination of sulfur with each carbon material, and determined what electrochemical property of the sulfur-cathode material increased in relation to the conductivity of the carbon matrix.
Graphite oxide was synthesized from natural graphite with a modified Hummer’s method [20]. Graphite, sodium nitrate and sulfuric acid were stirred; then potassium permanganate was added into the mixed composite while stirring vigorously. Next, H2O and H2O2 were added to the solution. The bright yellow, oxidized solution was washed with distilled water and HCl (43%). Graphite oxide powder was obtained after freeze-drying. GNS was prepared by the reduction of graphene oxide (GO) with sodium borohydride (NaBH4) as described elsewhere.
2.2. Synthesis of GNS/S and AC/S
GNS/S and AC/S composites were prepared by hydrothermal process, by which GNS, activated carbon (AC, MSP-20) and sulfur were dissolved in organic solvent (CS2). At first, we prepared 20 mL vials. Into each vial, we then poured 1 mL of CS2 into a mixture of 0.1 g of GNS, AC, and one of 10 wt%, 15 wt%, 20 wt%, or 40 wt% of sulfur. The mixture was stirred for 2 h and then sonicated at 25℃ for 1 h. After that, the mixture was heated at 30℃ to dry the solution. The dried GNS/S composites were heated at 155℃ to allow the sulfur to enter the GNS.
2.3. Morphological and physical measurement
The structure, morphology and nanostructure of the composites were examined by scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray diffraction (XRD). The quantity of sulfur was measured by thermogravimetric analysis (TGA) analysis.
2.4. Preparation of electrodes
The electrochemical qualities were measured by cyclic voltammetry (CV). We prepared the glassy carbon electrode (GCE) (working electrode). First, 5 mg of active material, 0.5 mL of distilled water and 0.1 mL of Nafion were sonicated for 1 h. Then, the GCE electrode was coated with 3 μL of the mixture. Then, the coated GCE electrode was dried at 100℃ for 30 min. Finally, we obtained a working GCE electrode.
2.5. Electrochemical measurement
All electrochemical tests were done in a three electrode system. We used a platinum rod as a counter electrode and a saturated calomel electrode was used as the reference electrode. We prepared the electrolyte by adding 0.5 M of LiPF6 as lithium salt, to dimethylformaide solution [21]. The mixture was stirred until the salt was dissolved completely.
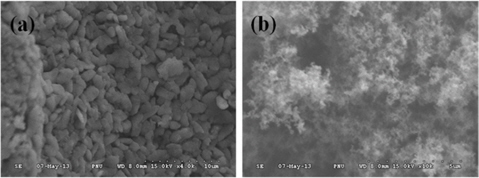
SEM images (Figs. 1a and b) were used to study the coated sulfur on the surface of the carbonaceous materials. As shown in Fig. 1a, the GNS/S composites are uniformly distributed over a wide area. The other SEM image (Fig. 1b) is AC (MSP-20) covered with sulfur particles. Also, the image in Fig. 1b confirmed that sulfur is as widely distributed on the AC in the GNS/S composites. The reason for this is that sulfur (which was dissolved in CS2 solvent) is easy to distribute uniformly on the GNS surface. Also, on heating, the viscosity of the sulfur melt slowly decreases, followed by a remarkable increase around 160℃ as a result of the opening and polymerization of the S8 rings. This condition persists until near 190℃, at which temperature sulfur starts depolymerizing and the viscosity decreases [22].
The sulfur (carried in CS2 solvent) coated onto the carbonaceous materials was better distributed because it melted by hydrothermal process at 190℃.
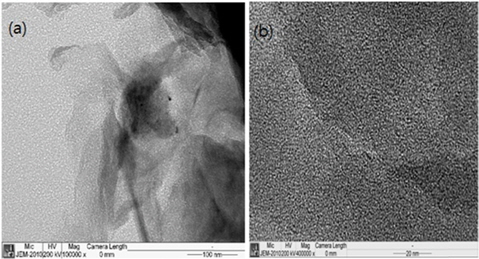
Figs. 2a and b, show TEM images of the GNS/S and AC/S composites. We can see the dynamically crystallized sulfur at room-temperature, and the melted sulfur at 190℃. The TEM image in Fig. 2a shows the edge of a graphene sheet (two-dimensional) with an anchored sulfur crystal. The TEM image in Fig. 2b shows the coated sulfur, which is indicated by the black area in the image.
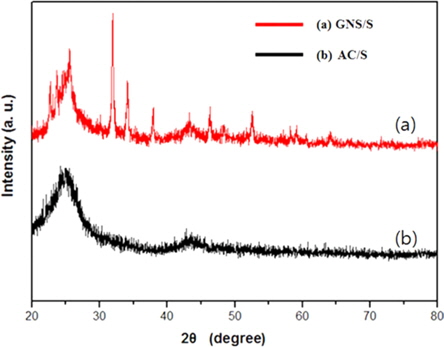
Fig. 3 shows the XRD patterns of the GNS/S and AC/S composites. All diffraction peaks of sulfur match the standard diffraction lines of sulfur, which could be indexed to the orthorhombic phase with a space group of Fddd [1]. For the GNS/S and AC/S composites, the XRD pattern consists of diffraction peaks of sulfur and a broad diffraction peak at 25°.
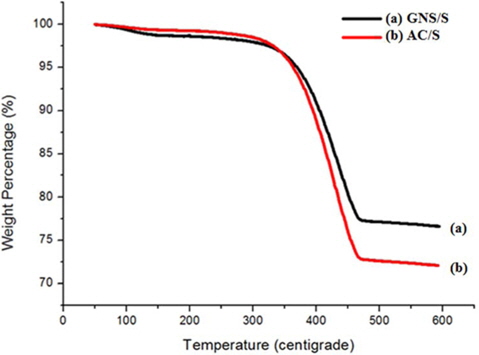
Fig. 4 demonstrates the TGA curve of the GNS/S and AC/S composites. When heated from 0 to 600℃, at a rate of 10℃/ min, the evaporation rate of the sulfur from the GNS/S and AC/S composites can be used as indirect evidence to locate the sulfur loaded onto the GNS and AC. As can be seen from Fig. 4, the AC/S and GNS/S composites started to oxidize at 350℃ and 250℃, respectively. After that, second mass change occurred at 450℃ and 350℃.
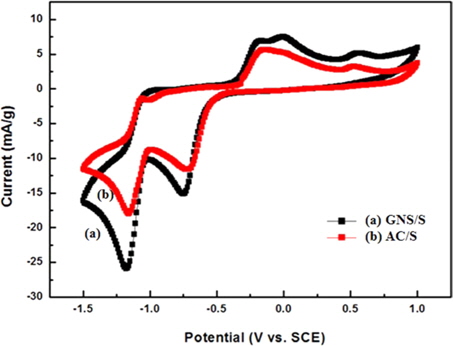
Fig. 5 shows the CV curves of the GNS/S- and AC/S-composite electrodes in the three-electrode system. We measured from -1.5 V to 1.0 V, at 10 mV/S, to check the oxidation and reduction peaks. We carried out tests for 50 cycles. In the initial cycle, both AC/S and GNS/S composites did not show properties of CV.
The composites show oxidation and reduction peaks after achieving electrode stability. For the CV of the AC/S composite, during the first cathodic-reduction process, two peaks (at about −0.5 and −1.0 V) were observed. The oxidative peak between −0.2 and 0.4 V indicated a lower-ordered polysulfide (e.g., S8 to Li2S8 process). At first, higher-ordered poly-sulfides were indicated between 0.5 and −1.0 V (such as Li2Sx, 1 ≤ x ≤ 4), and later, higher-ordered poly-sulfides were indicated between −1.0 and −1.5 V (such as Li2Sx, 4 ≤ x ≤ 8).
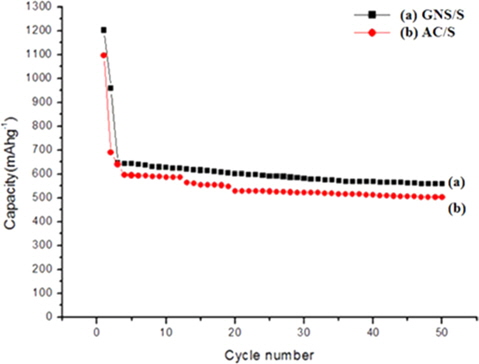
Fig. 6 shows the discharge capacity versus cycle number of the GNS/S and AC/S composites. The GNS/S contains 20 wt% sulfur. It was found that the initial discharge capacity of the GNS/S and AC/S composites were 1100 and 1211 mAh g−1, respectively, at a current density of 50 mA g−1.
The initial capacity of the GNS/S electrode was higher than that of the AC/S electrode. Furthermore, the cycling stability of the GNS/S composite was also improved. The improvement in the capacity and cycling stability of the electrode with the GNS/S composite electrode may be because the graphene in the composite improved the conductivity of the electrode.
In this study, GNS/S and AC/S composites was prepared using the infiltration method with organic solvent. Using organic solvent enabled high capacity because it allowed uniform distribution of the melted sulfur on the carbon-materials. Also, by comparing carbon materials with GNS, which has higher conductivity than AC, we concluded that the electrochemical property of the sulfurcontaining GNS electrode had been improved due to the fact that the graphene has higher conductivity and better dispersion. This enabled an increase in the effective surface area of the composite electrode. The graphene-sulfur composite cathodes show excellent electrochemical performance with high specific capacity and good stability in performance, compared to the activated carbon-sulfur composite. These results demonstrate that these GNS/S cathodes are promising candidates for lithium-sulfur cells.