Various energy storage devices have been developed to address the pressing demand for high-energy, high-power-density and long-life energy storage devices for applications ranging from portable electronics to biomedical devices to hybrid electric vehicles. Among these devices, electrochemical double-layer capacitors (EDLCs), commonly known as super-capacitors, have emerged as a promising energy storage option for applications that require high power along with exceptional storage and cycle lives [1].
Carbonaceous materials are a practical electrode material for EDLCs because of their high specific surface area, proper pore size distribution, good electronic conductivity, good chemical stability, low cost of economic production, and easily tuned morphology and porosity [2,3]. The porous properties of activated carbons are well known to strongly depend on the structure of their precursor (Bansal
However, the micropores of activated carbons limit accessibility to electrolytes during the charge/discharge process and are disadvantageous for ion diffusion, particularly under high-loading current-density conditions. Moreover, the mesopores of the activated carbons have a relatively low specific surface area, accompanied by low capacitance. For these reasons, the synthesis of hierarchical-porous carbon with both micropores and mesopores is important for EDLC performance because micropores and mesopores are complementary [5,6].
The template technique is an important method for achieving various pore sizes and structures in the micropore to macropore range and is typically accomplished by changing the template of the carbon precursor. Importantly, silicon dioxide (SiO2) acts as a physical template to control the size and morphology of the final product and leaves no trace such as the chemical composition in the final product. Additionally, SiO2 and be used to aid in the manufacture of mesoporous carbon by acting as a SiO2 template [7,8].
In the present study, pore control of novolac-type phenol-based activated carbon with a hierarchical pore structure (micropores and mesopores) was improved using a SiO2 template with different SiO2:novolac resin weight ratios for physical activation and 4 M potassium hydroxide (KOH) as an chemical activating agent. The effects of physical and chemical activation on the surface morphology and textural properties of the novolac-type phenol-based activated carbon were investigated, and products were evaluated as potential electrode materials in EDLCs.
Novolac (phenol formaldehyde resin, KPH-F2004, Kolon) was melted in an organic solvent such as alcohol or acetone. Because novolac has a linear structure that consists of linked methylene groups (-CH2-), methanol (methyl alcohol, Samchun) was used as the solvent for mixing novolac and SiO2 (Degussa, R972, average size = 16 ± 2 nm). To generate mesoporous carbon materials, SiO2 was used as the template for physical activation and hydrofluoric (HF acid, 48.0%-51.0%, J.T Baker) was used for silica removal. Additionally, chemical activation was performed using KOH (95.0%, Samchun) to obtain microporous carbon materials.
2.2. Preparation of silica-embedded novolac solution
The SiO2 and novolac dissolved in methanol were mixed with an internal mixer at 45°C until entirely melted and homogenized. Sonication was used to disperse SiO2 within the melted solution. The SiO2 recruitment stock was 5, 15, and 30 wt% compared to novolac.
Before carbonization, the mixture solution of SiO2, novolac, and methanol was cured at 150°C for 4 h under air. Consequently, cured samples of materials that had originally exhibited thermoplastic characteristics now exhibited thermosetting characteristics. The heating rate was 1°C/min during this step. The cured samples were subsequently carbonized under a nitrogen atmosphere at 1050°C for 1 h. During this step, the heating rate was 5°C/min.
To form mesoporous novolac-type phenol based carbon, carbonized products were soaked in HF for 24 h and washed with distilled water several times. Finally, each sample was dried at 120°C overnight. During this process, SiO2 in the novolac was removed via the reaction:
2.5. Chemical activation using KOH
A hierarchical porous novolac-type phenol based-activated carbon materials were prepared from the mesoporous carbon materials using KOH. We chemically activated the mesoporous phenol-based carbons by dipping 1 g of each sample in 20 mL of 4 M KOH for 4 h. The mesoporous phenol-based carbons in this KOH solution were subsequently treated at high temperatures under flowing nitrogen. The temperature during this process was maintained at 750°C for 3 h. After chemical activation, the resulting carbon samples were washed with distilled water to remove residual potassium and dried at 120°C overnight.
The carbonized phenol-based carbon and hierarchical-porous (micropores and mesopores) structured materials were prepared using various weight ratios of SiO2 template (0, 5, 15, and 30 wt%) relative to novolac as follows: CN, 5SNK, 15SNK, and 30SNK.
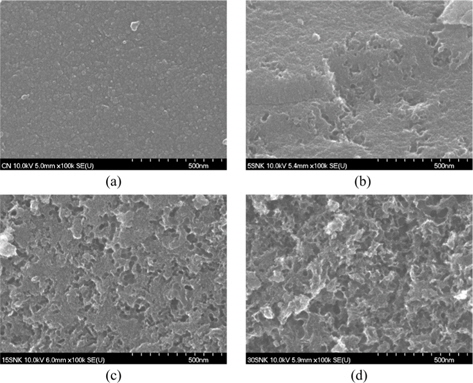
The surface morphology of developed hierarchical porestructured carbon materials were investigated using field emission scanning electron microscopy (FE-SEM; Hitachi, S-5500, KBSI).
The textural properties of the prepared samples were also investigated. Each sample was degassed at 150°C for 180 min, and nitrogen adsorption was conducted at 77 K using a Micromeritics ASAP 2020 volumetric adsorption apparatus to study the pore characteristics such as specific surface area, total pore volume, and pore size distribution.
For the preparation of EDLC electrodes, 80 wt% prepared samples, 10 wt% carbon black (Super P; Timcal Ltd., Switzerland), and a 10 wt% solution of polyvinylidene fluoride (PVDF; Aldrich, USA) were mixed in N-methyl pyrrolidone (NMP; Aldrich, USA) to form a slurry. The slurry was subsequently painted onto a titanium plate. Electrochemical characterization of the hierarchical pore structured (micropores and mesopores) phenol-based carbon was conducted with a computer-controlled potentiostat-galvanostat (Ivium Technologies, The Netherlands) using a three-electrode assembly.
In the three-electrode assembly, the hierarchical porous phenol- based carbon, a Ag/AgCl electrode, and a platinum plate were used as the working, reference, and counter electrodes, respectively. In addition, cyclic voltammetry (CV) was performed over a potential range of 0-1 V at a scan rate of 5 and 50 mV/s; 1 M H2SO4 was used as the electrolyte for the electrochemical measurements.
3.1. Surface morphology of phenol-based activated carbon with hierarchical pore structure
To examine the surface morphology, carbonized phenol-based carbon and hierarchical porous phenol-based carbon were studied by SEM. As shown in Fig. 1, pores with different sizes and different morphologies existed within the structure of the prepared samples. Chemical activation with KOH influenced the surface roughness of the carbon materials as a consequence of reactions between the carbon surface and KOH. Surface carbon was consumed while pores were developed by opening previously closed surface pores during the gasification process [9-11]. The increase in surface roughness was confirmed to result in chemical activation, as shown in Figs. 1a and b. In addition, Figs. 1b and d present SEM images of the physical and chemical activated carbons prepared using different amounts of the SiO2 template. After the HF treatment, the SiO2 template formed around openings of a fixed size, comparatively. The portion of round openings broadened with increasing quantity of SiO2. The surface roughness of 30SNK, in particular, almost completely disappeared.
3.2. Textural properties of phenol-based activated carbon with hierarchical pore structure
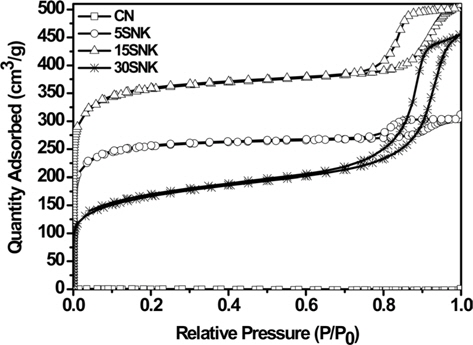
Fig. 2 shows nitrogen sorption isotherms of the carbonized carbon and physically/chemically activated carbon; the sorption measurements were performed at 77 K. On the basis of the International Union of Pure and Applied Chemistry (IUPAC) classification scheme, the isotherm of the physically/cemically activated carbon was Type IV [12,13]. The activated carbon samples exhibited a dramatic increase in the quantity of nitrogen adsorbed below 0.01 P/P0, indicating the development of a microporous structure; this effect was most pronounced in the case of 15SNK [14-16]. The isotherms of the activated carbon increased markedly below 0.1 P/P0 and then increased slowly above 0.1 P/P0, indicating the development of micropores and some mesopores in the activated carbon due to chemical activation by KOH and physical activation by the SiO2 template, respectively. Hysteresis loops were observed in the cases of 5SNK, 15SNK, and 30SNK, which were indicated to contain mesopores with diverse shapes, including bottle-necks, wedges, etc. The increase in SiO2 content resulted in mesopores of diverse shape after the HF treatment. The width of the isotherm hysteresis loop gradually increased in proportion to the amount of SiO2 template used [17,18].
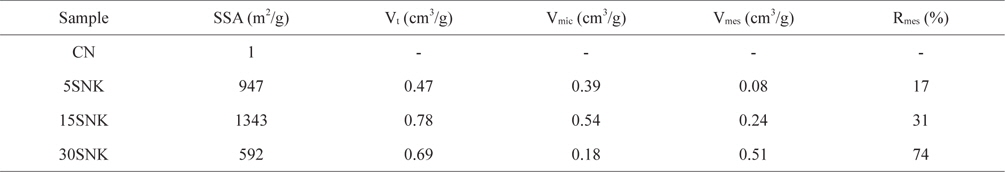
The textural properties of novolac-type phenol-based activated carbon extracted from the Brunauer-Emmett-Teller (BET) equation and the T-plot method are listed in Table 1. The specific surface area of CN (1 m2/g), 5SNK (947 m2/g) and 15SNK (1343 m2/g) increased with increaseing SiO2 content, whereas that of 30SNK (592 m2/g) decreased under similar circumstances. The micropores provide a high surface area. Additionally, the SiO2 caused aggregation and resulted in less-developed micropores after KOH activation because of a decrease in the number of active sites in the case of 30 SNK. The well-dispersed SiO2 generated numerous active sites after the HF treatment and reaction KOH. Thus, 30SNK was activated inefficiently by KOH and contained less-developed micropores, whereas the number of micropores in 5SNK and 15SNK increased. However, the mesopore volume of all the samples increased with increasing SiO2 content. These results indicate that the amount of SiO2 was an important factor for pore properties.
[Table 1.] Textural properties of phenol-based activated carbons with hierarchical pore structure
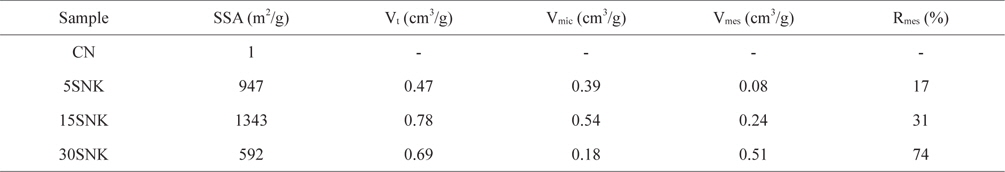
Textural properties of phenol-based activated carbons with hierarchical pore structure
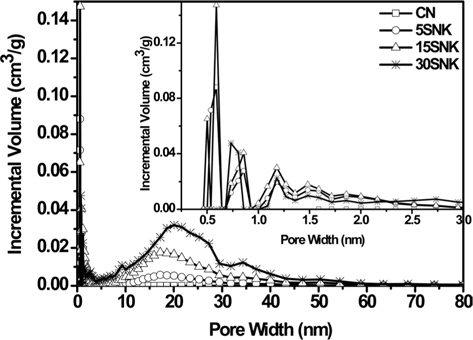
Fig. 3 shows the pore size distributions of the prepared samples before and after physical and chemical activation, as calculated via the density functional theory method. Micropores smaller than 3 nm developed extensively in 15SNK, and the other samples have a similar range of micropores, with little difference in pore volume after KOH activation [19]. However, in 30SNK, mesopores between 5 and 50 nm were mostly formed as a consequence of SiO2, aggregation; the mesopore sizes of the other samples decreased in the order 15SNK > 5SNK > CN. In all of the samples, the most common mesopore size was 15-20 nm.
3.3. Electrochemical properties of phenol-based activated carbon with hierarchical pore structure
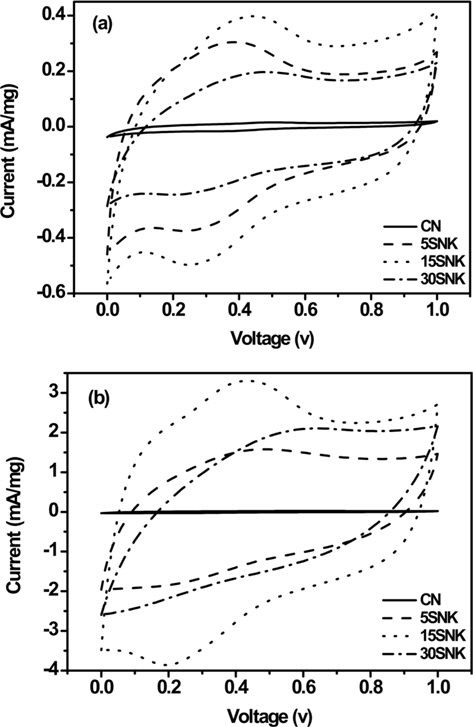
Fig. 4 provides the CV of the novolac-type phenol-based activated carbon within a potential range of 0-1.0 V at scan rates of 5 and 50 mV/s. At a scan rate of 5 mV/s (Fig. 4a), the shapes of the CV profiles of the samples were approximately rectangular, and redox peaks were observed at 0.3-0.4 V. At the higher scan rate (i.e., 50 mV/s), the profiles became gradually depressed (Fig. 4b). We attributed such tilted rectangular shapes to the influence of the ohmic resistance from the electrolyte motion in the carbon pores upon double-layer formation [20]. These rectangular shapes likely arise because the ions can transport into pores more easily at a low scan rate than at a high scan rate [21,22]. This phenomenon was prevalent in CN, 5SNK, and 30SNK, though minimally in 15SNK. We interpreted these results as indicating that the coexistence of micropores and mesopores with a high specific surface area provides ion accessibility to an electrode surface at a wide range of scan rates.
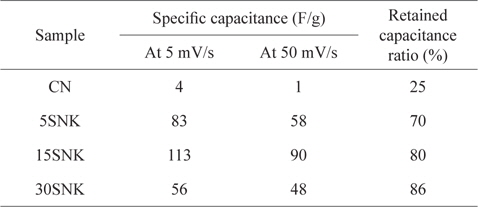
The specific capacitances of the prepared EDLCs are shown in Table 2. The 15SNK exhibited a higher specific capacitance at both scan rates (5 and 50 mV/s) compared to those of the other samples. The specific capacitances of 15SNK were 113 and 90 F/g at scan rates of 5 and 50 mV/s, respectively. 30SNK exhibited the highest retained capacitance ratio; however, its specific capacitances of 56 and 48 F/g at scan rates of 5 and 50 mV/s, respectively, were lower than expected. These results indicate that 31% of the mesopore volume ratio is appropriate for both improved specific capacitance and retained capacitance ratio. A greater mesopore volume ratio results in decreased specific surface area and specific capacitance. The highest specific capacitances and greatest enhanced retained capacitance ratio were observed in the case of 15SNK. Therefore, increases in the micropore volume, mesopore volume, and specific surface area resulted in a higher specific capacitance and enhanced retained capacitance ratio, suggesting considerably improved EDLC performance.
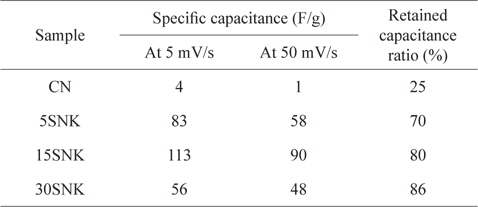
The specific capacitances of phenol-based activated carbon electrodes at scan rates of 5 and 50 mV/s
In this study, phenol-based activated carbon with a hierarchical pore structure was prepared by physical and chemical activation using a SiO2 template and KOH; the activated carbon was designed for enhanced EDLC performance. By studying the morphology of the activated carbon with a hierarchical pore structure, we observed that the roughness of the surface of the activated carbon increases with increasing SiO2 content, as determined via SEM observations. The BET specific surface area and micropore volume of 15SNK were the highest among the tested samples: 1343 m2/g and 0.54 cm3/g, respectively. For all of the samples, the mesopore volume increased approximately 74% when the SiO2 content was increased. In the case of 30SNK, its specific surface area decreased despite having the highest SiO2 content due to the aggregation of SiO2. Through CV, we confirmed that the developed micropores and mesopores with high specific surface area improved the specific capacitance of the resultant activated carbon electrode even at high scan rates: 113 and 90 F/g at scan rates of 5 and 50 mV/s, respectively. Additionally, the retained capacitance ratio of 15SNK was enhanced compared to those of CN and 5 SNK. Therefore, physical and chemical activation using a SiO2 template and KOH is an effective method for improving the specific capacitance and retained capacitance ratio of activated carbons.