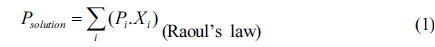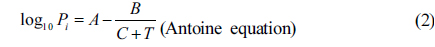A porous silicon microcavity (PSMC) sensor has been made for vapors of solvent solutions, and a method has been developed in order to obtain simultaneous determination of two volatile substances with different concentrations. In our work, the temperature of the solution and the velocity of the air stream flowing through the solution have been used to control the response of the sensor for ethanol and acetone solutions. We study the dependence of the cavity-resonant wavelength shift on solvent concentration, velocity of the airflow and solution temperature. The wavelength shift depends linearly on concentration and increases with solution temperature and velocity of the airflow. The dependence of the wavelength shift on the solution temperature in the measurement contains properties of the temperature dependence of the solvent vapor pressure, which characterizes each solvent. As a result, the dependence of the wavelength shift on the solution temperature discriminates between solutions of ethanol and acetone with different concentrations. This suggests a possibility for the simultaneous determination of the volatile substances and their concentrations.
Owing to high specific surface area [1], porous silicon (PS) is an ideal transducer material for sensors of liquids [2-4] and vapors [5, 6]. Recently, PS optical sensors have been designed in the structure of one-dimensional photonic crystal devices such as optical filters [7] and microcavities [8]. The principle of these sensors is a determination of the photonic crystal spectral shift caused by refractive index change of the nano-porous silicon layers in the device due to the interaction with liquid or gas. It is shown from the principle that the response of the sensor is only dependent on refractive index and therefore lacks specificity for the studied substances. Consequently, most of current sensors based on the PS photonic crystal only determine the concentration of a defined substance. It is possible to use a physical or chemical method to overcome this drawback. A commonly used chemical method is the functionalization of the surface of silicon nanocrystals in the porous layers [9, 10]. It is a chemical process to create the new chemical bond that combines selectively with molecules of the studied substances. The number of published works, which used a physical method to identify the analyses, in sensors based on photonic crystals has been limited. Sailor applied temperature cycles to a porous silica photonic crystal embedded in pure chemical vapors and therefore he was able to distinguish between isopropanol, heptane, and cyclohexane [11], Ye presented a multi-beam interferometry method using optical fibers for detection of methane gas with concentration sensitivity of 0.015% [12]. The sensitivity of optical sensors is defined as the ratio of the wavelength shift and the change of the ambience refractive index, and depends on the concentration change of the solution, can be enhanced by designing suitable structural parameters such as thickness, porosity, number of porous layers in the device [13] or creating a stress on the sensor surface [14].
This paper presents the use of a vapor sensor based on a porous silicon microcavity for the determination of solvent solutions. We set up the measurement, in which the temperature of the solution and the velocity of the airflow containing the solvent gas from solutions of ethanol and acetone control the response of the sensor. As mentioned above, the sensor uses the physico-chemical properties of the analyzed substances as “characteristic signals” involved in the response of the sensor. The sensor response is given by the shift of the resonant wavelength of the microcavity when sensors are immersed in the flow of solvent vapor. We present a study of the dependence of the wavelength shift on solvent concentration, velocity of the airflow and solution temperature. Considering those dependencies, we hope to find out the potential to enhance the sensitivity of the sensors and the specificity of the measurement.
Porous silicon microcavities were fabricated by an electrochemical method in a process that was presented in our previous work [15]. In particular, the electrochemical process was carried out on a (100)-oriented boron-highly doped p-type Si wafer (resistivity is of 0.01-0.1 Ω.cm) in a 16% hydrofluoric acid (HF) solution and ethanol at various current densities. Aluminum was evaporated onto the backside of the Si wafer and then it was annealed at 420℃ in nitrogen atmosphere for 45 min. in order to ensure a good Ohmic contact. The electrochemical process was controlled by computer program using Galvanostat equipment (Autolab PGSTAT 30) so precise control over electrical current and etching time was achieved. Before electrochemical etching, the Si wafer was dipped in 5% HF solution for a minute to remove the native oxide. The electrochemical anodization cell was made of polytetrafluoroethylene (Teflon) resin and was designed to have an exposed etching area of approximately 0.79 cm2. After anodization, the sample was washed with 98% ethanol and dried in primary vacuum. For converting the surface of the silicon nano-crystals from hydrophobic to hydrophilic, we oxidized the as-prepared sample in an ozone atmosphere for 45 min. by using the ozone generator (H01 BKOzone with a capacity of 500 mg/hour).
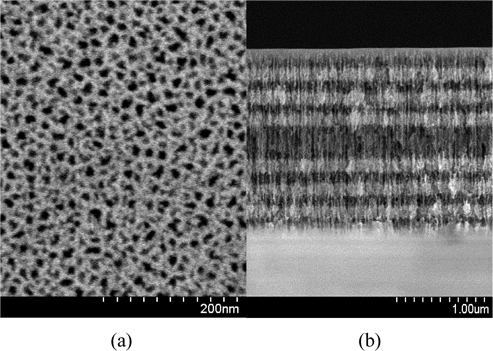
Cross-sectional and top view images of the porous silicon microcavity were obtained using an ultra resolution field-emission scanning electron microscope (FE-SEM) S-4800. Figure 1 shows plan-view and cross-section images of the microcavity based on (HL)3,5LL (HL)3 porous silicon multilayer structure, where H and L labels correspond to high and low refractive index layers, respectively, 3.5 means three and half pairs of HL. We choose structure with 3 and 3.5 pairs of HL, because this gives a good reflectivity spectrum and easily repeatable electrochemical etching process. The thicknesses of high and low refractive layers were 72 nm and 87 nm with accuracy of ± 2 nm, respectively. This structure was obtained from anodization current density of 15mA/cm2 and 50mA/cm2 and with etching time 5.56 sec and 2.86 sec for high and low refractive index layers, respectively. For measurement of reflective spectra of the samples, we used a spectrometer (S-2000, Ocean Optics) with a resolution of 0.4 nm and a tungsten halogen lamp (Z 19, Narva).
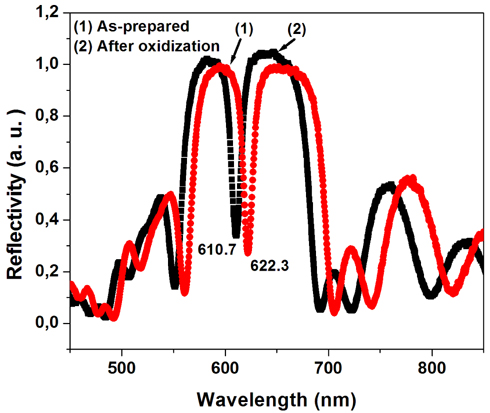
Figure 2 shows the reflectivity spectra of the microcavity before and after oxidization. The blue shift of the resonant wavelength after oxidization is due to a decrease in the effect of refractive index of the porous layers in the microcavity [16]. From experimental results we calculated refractive indices of 2.1 and 1.75 for high and low refractive index layers, respectively.
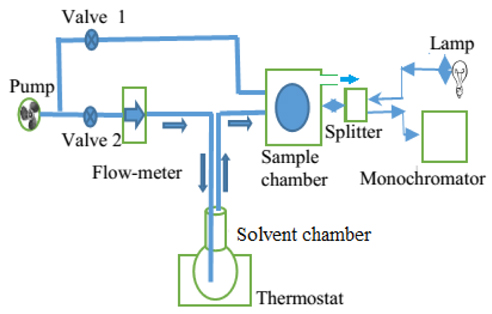
Figure 3 shows the schematic of the concentration measurement for volatile organics by using a vapor sensor based on a porous silicon microcavity. In this schematic, valve 2 works as a controller of velocity of air stream though the flow meter, the test solvent chamber and the sample chamber. Valve 1 is only open to refresh the porous matrix after measurement. The thermostat controls the temperature of the liquid in the range from room temperature to 100℃.
In our experiment, we use an optical fiber splitter BIF200 UV-VIS for light irradiation to samples and for collecting the reflective spectrum of the microcavity. We have also used a LM35D integrated circuit for measuring the temperature in the sample chamber. It is shown that this temperature was not affected by the solution temperature nor by the air rate in our experimental setup. For an experimental data run, it takes 5 min. to 7 min. depending on the velocity of the airflow. A standard deviation of the wavelength shift from the average value of 5 experimental data runs is 0.6 nm.
It is known that the response of the sensor depends on solvent vapor pressure in the sensor chamber [17]. This vapor pressure is related to the vapor pressure of the solvent in the solution chamber through a gas stream flowed through the solution. Assuming that the vapor pressure in the solution chamber obeys the rules of vapor pressure in a closed system, the relation between the wavelength shift (
Where
The equations (1-2) show that
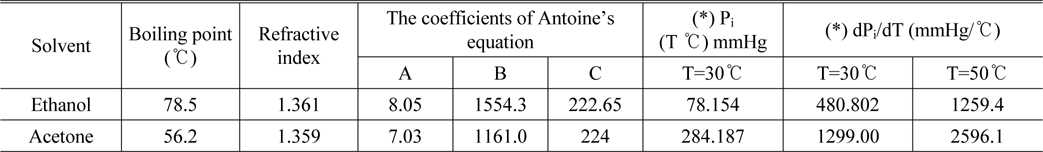
As mentioned above, we carried out experiments on ethanol and acetone solution. These are very common organic solvents and some of their physical properties such as boiling point, refractive indices and Antoine’s coefficients from [18] are shown in Table 1.
[TABLE 1.] Physical properties of acetone and ethanol
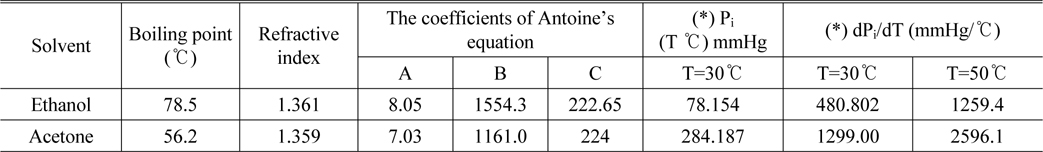
Physical properties of acetone and ethanol
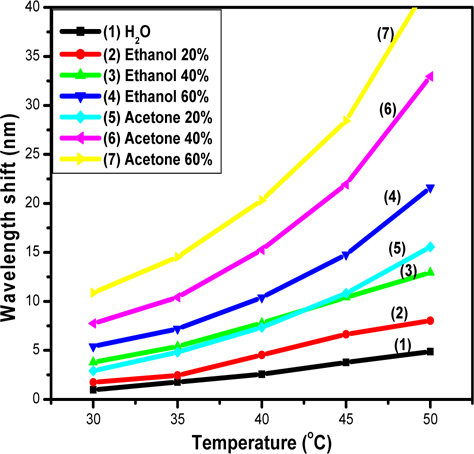
Figure 4 shows the dependence of
The slope of
As our previous work [4], we have tested the response of the sensors immersed to liquid phase solution of 100% acetone and denatured ethanol using a Cary 5000 spectrometer system with a resolution of 0.1 nm. A difference of 0.5 nm in the resonance wavelength shift of cavity for these two solvents was obtained due to the small difference in their refractive indices.
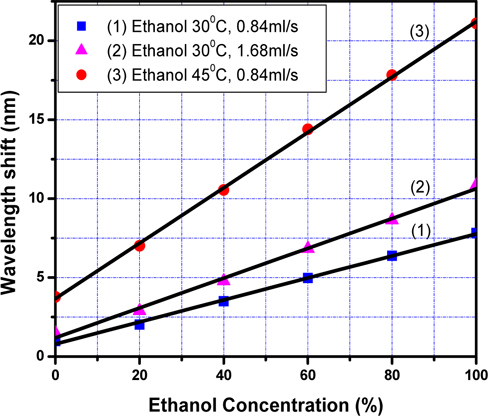
Figure 5 shows the dependence of the resonant wave-length shift
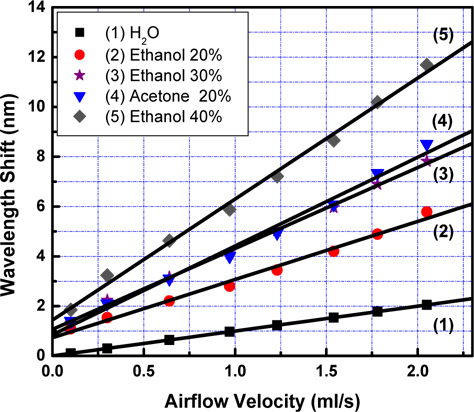
Figure 6 shows the dependence of Δλ on V,
We also apply those results for curve 2 and 4 received from measurements with ethanol concentrations at 40% and 20%. Slope of curve 4 is larger than that of curve 2 due to the greater value in concentration. It is deduced from Fig. 6 that dependence of the wavelength shift on velocity of the airflow is linear, and the slopes
In conclusion, we successfully built a high sensitivity measurement system for the determination of solvent solutions by using a vapor sensor based on a porous silicon microcavity. In this measurement, the sensor response is controlled by temperature of the solution and velocity of the air stream flowing though the solution. We studied the dependence of the wavelength shift on solvent concentration, velocity of the airflow and temperature solution for the solutions of ethanol and acetone with various concentrations and in order to enhance the sensitivity and specificity of the measurement. The dependence of the wavelength shift on concentration is linear and sensor sensitivity increases with temperature of the solution and velocity of the air stream. Solution temperature and air flow velocity determine the equilibrium of partial vapor condensation in the pores and then contain characteristics of specific solvent vapor pressure and liquid refractive index that allows discrimination between ethanol and acetone and determine the concentration. This suggests a possibility for simultaneous determination concentration and type of solvent.