Bang-poong-tong-sung-san (BPTS) is a traditional oriental prescription, which is described in Sun-myungnon- bang, a classical literature of the oriental medicine. BPTS was widely known to be effective in stroke, hypertension, arteriosclerosis, constipation and cutaneous disease. It was also reported that BPTS has effects on obesity and hyperlipidemia. The prescription of BPTS is composed of 18 medicinal herbs as follows;
The marker compounds of BPTS were 24 components proposed by Korea Institute of Oriental Medicine (Daejeon, Korea). The therapeutic effects of BPTS were influenced by many complex components of the prescription. In order to investigate the effect of BPTS, the nature of the various components in the prescription is very important. The chemical profile and pharmaceutical efficacy of BPTS may be significantly affected by geographical sources, harvesting, processing and storage of medicinal herbs. Therefore, the quality control method for the marker compounds of BPTS is needed to assure its efficacy and quality.3
In the past few years, hyphenated mass spectrometry techniques have developed for the identification of the bioactive compounds in traditional Chinese medicine prescription. It was mainly used liquid chromatographytandem mass spectrometry (LC-MS/MS),4-6 LC-ion trap MS,7,8 LC-time-of-flight (TOF) MS9,10 and quadrupole- TOF (Q-TOF) MS.11,12 These mass spectrometry techniques are useful due to identify components selectively in complicated herbal matrix. IT-TOF MS used in this study has merits which can simultaneously measure accurate masses of analytes and perform MSn analysis. Thus identification of compounds could be accurate. The analytical research of BPTS components was not previously carried out.
In this study, we conducted development of qualitative detection method and chemical profiling of 19 marker compounds of BPTS using LC/UV-IT-TOF MS system. The hybrid ion-trap time-of-flight (IT-TOF) mass spectrometry is a powerful tool for screening and identification. The MSn (n = 1~10) ability of the ion-trap mass spectrometry gives more structural information than triple quadrupole mass spectrometry, and the time-of-flight (TOF) mass spectrometry can obtain accurate mass. Thus, IT-TOF gives more accurate structural information about precursor and product ions by adopting MSn analysis and accurate mass measurement.
To the best of our knowledge, this is the first study on the integrated chemical identification of the BPTS preparation and could help quality control and understand of relationship of efficacy and chemical profile.
The BPTS extract powder and the marker compound standards (ephedrine, albiflorin, paeoniflorin, paeonol, nodakenin, decursin, decursinol angelate, liquiritin, isoliquiritin, isoliquiritigenin, liquiritigenin, glycyrrhizin, 6- gingerol, sennoside A, menthol, menthone, geniposide, baicalin, baicalein, wogonin and wogonoside) were provided from Korea Institute of Oriental Medicine (Daejeon, Korea). Glacial acetic acid (99.7%), formic acid (98%) and ammonium formate were purchased from Sigma Aldrich (St. Louis, MO, USA). High-performance liquid chromatography (HPLC)-grade water was obtained from Fisher Scientific (Fair Lawn, NJ, USA). HPLC-grade methanol and acetonitrile were from J. T. Baker (Phillipsburg, NJ, USA). All other chemicals used were analytical grade.
>
Standard and sample preparation
All standard reference powder of marker compounds were dissolved in dimethylsulfoxide (DMSO) at appropriate concentration and mixed all. Then, diluted with 50% methanol. A standard mixture solution was prepared for 50 μg/mL of each compounds except for 500 μg/mL of paeonol.
Extraction was performed by adding 10 mL of methanol to 0.1 g of the provided BPTS extract powder, vortexing for 10 min and sonicating for 30 min. The extracted solution was centrifuged at 4,000 rpm for 10 min. The supernatant was filitered by a 0.45 μm nylon filter, and the filtrate was injected to LC/UV-IT-TOF MS.
>
Instrumentations and separation conditions
The marker compounds of BPTS were analyzed by Shimadzu Prominence LC-20 series HPLC system with ITTOF MS spectrometer equipped with an electrospray ionization source (Shimadzu, Kyoto, Japan). The chromatographic separation was performed by Capcell Pak C18 column (1.5 mm × 250 mm, 5 μm, Shiseido, Tokyo, Japan). The temperature of the column and autosampler was kept at 40℃ and 10℃, respectively. All marker compounds showed optimized sensitivity and resolution at 0.1% formic acid for aqueous part of mobile phase when compared with 0.1% formic acid, 0.1% acetic acid and 5 mM ammonium formate. Gradient elution of the mobile phase was composed of a 0.1% formic acid aqueous solution (solvent A) and acetonitrile (solvent B). The flow rate was 0.1 mL/min with the following gradient program: 0% (B) for 0-10 min, linear gradient to 50% (B) for 10-150 min, 50-100% (B) for 150- 150.01 min, holding at 100% (B) for 150.01-160 min, and then returning to 0% (B) for column equilibration. The total run time was 175 min. The sample injection volumn was 2 μL. The wavelength of UV detector were 230 and 254 nm. The mass spectrometry was calibrated daily by use of sodium trifluoroacetate solution. The HPLC and mass spectrometry were controlled by LabSolution (Ver. 3.1.360) software (Shimadzu, Kyoto, Japan).
The MS detection was conducted in positive and negative ion mode for all compounds in scan range of m/z 100-1000. All marker compounds were used MS data in positive ion mode except for sennoside A. Capillary voltages were +4.5 kV (positive ion mode), and -3.5 kV (negative ion mode) and heat block temperature was 200℃. Nebulizing gas (N2) flow was 1.5 L/min and MSn analysis was automatic MSn (n = 1-3) by order of intensity. The precursor ion was selected over the range of m/z 100 to 1000 and the MSn spectra were obtained at the relative collision energy of 150%. Three precursor ions were selected automatically by order of their intensity (over 100,000 cps). Ion accumulation time in the ion trap was set to 10 ms for both MS and MSn (n = 2~3) mode. Data acquisition, processing and prediction of chemical formula were performed by LCMS solution (Ver. 3.60) software package (Shimadzu, Kyoto, Japan). The MS2 and MS3 libraries were built by library editor software included in LCMS solution software.
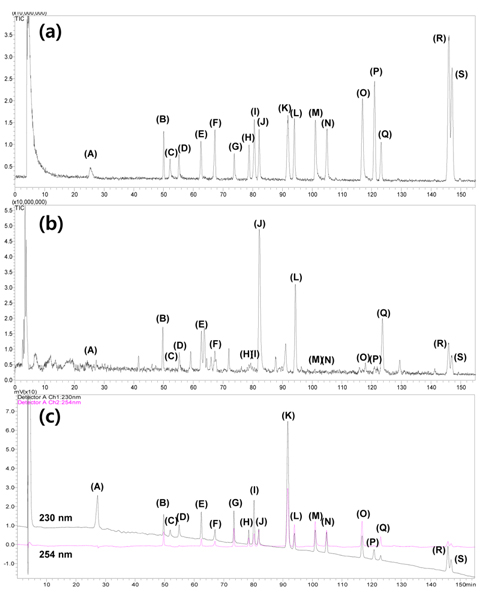
We developed LC/UV-IT-TOF mass spectrometry method for the qualitative analysis of BPTS extract and marker components identification was carried out by comparing with accurate mass of the molecular ion, and the fragment ion and retention time of the standard reference. This high accuracy mass spectrometry was enable to distingush of tiny mass difference of the compounds and to identify easily. The 19 standard compounds out of 21 marker compounds in BPTS favorably detected as follows; ephedrine, albiflorin, paeoniflorin, nodakenin, decursin, decursinol angelate, liquiritin, isoliquiritin, isoliquiritigenin, liquiritigenin, glycyrrihizin, 6- gingerol, sennoside A, geniposide, baicalin, baicalein, wogonin and wogonoside. The menthol and menthone were not detected in mass spectrometry due to their high volatility. The molecular ion peaks of all marker compounds were found in positive ion mode. While that of sennoside A was not detected in positive mode due to in-source collision. Because we could find molecular ion [M-H]− of sennoside A in negatve ion mode, we used identification data of sennoside A in this detection mode. MSn analysis was produced up to MS3. The values of mass error were almost obtained within less than 20 ppm in standards and BPTS extract samples. In the HPLC/UV chromatogram, 19 standard marker compounds were better detected in 230 nm than 254 nm. The total ion chromatogram of IT-TOF and UV chromatogram are shown in Figure 1.
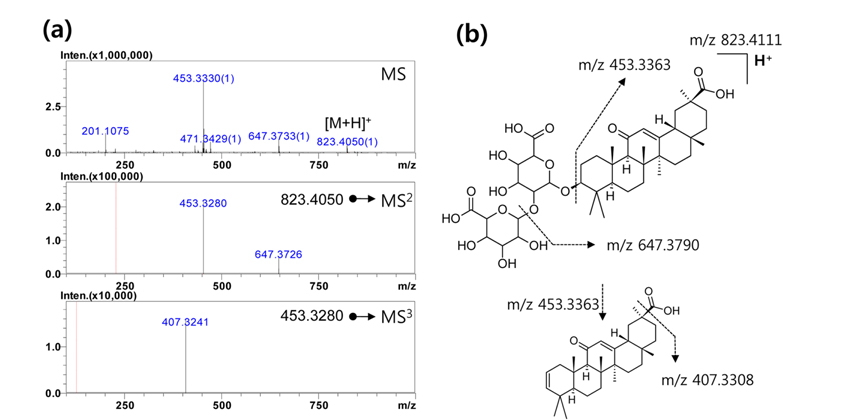
For example, the MS spectra and the fragment pattern of chemical structure for glycyrrhizin in positive ion mode are demonstrated in Figure 2. Glycyrrhizin is a marker compound of
In the methanol extract of BPTS, 17 marker compounds were detected except for paeonol and sennoside A when applied with a optimized condition of the LC/UV-IT-TOF mass spectrometry. We confirmed that baicalin and wogonoside are present in high concentration in BPTS extract. They are marker compounds of
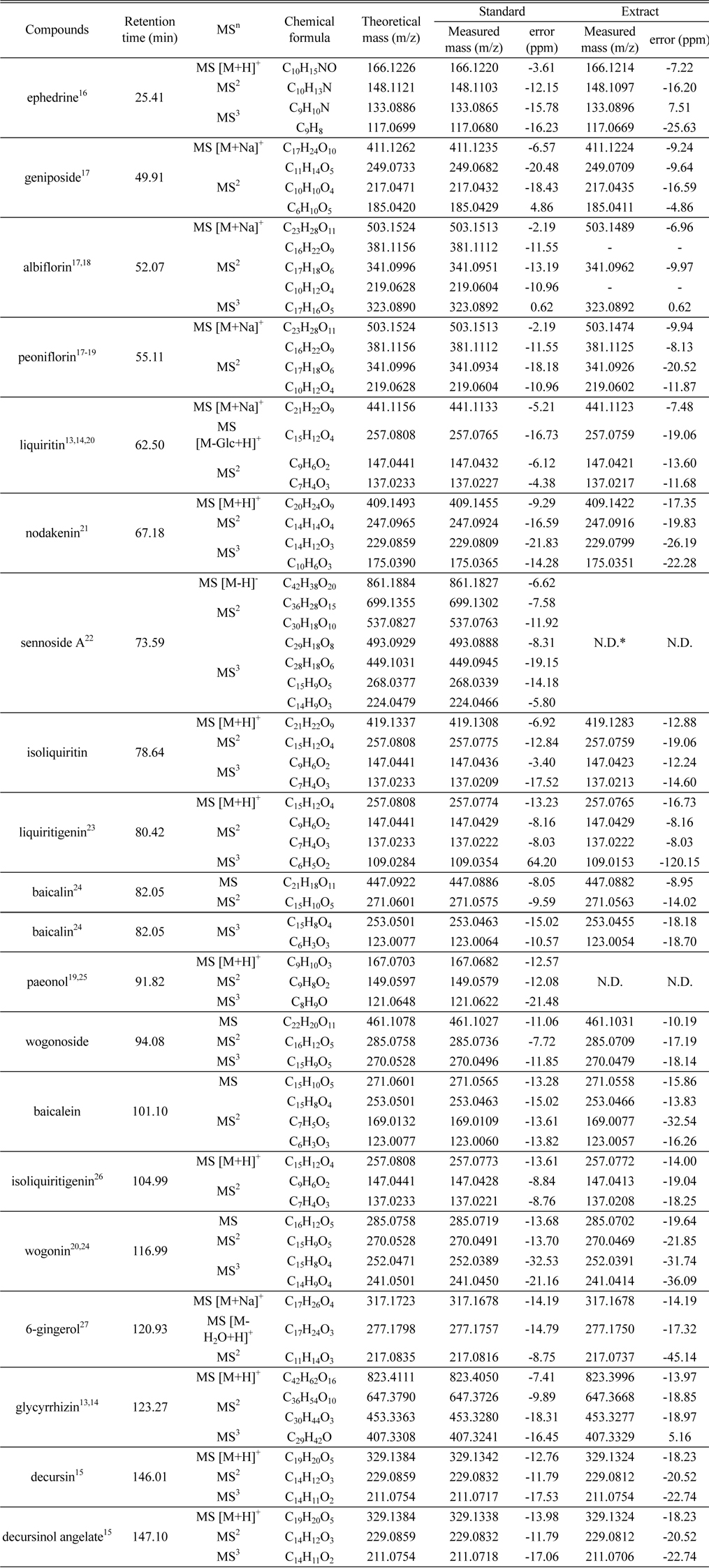
The chemical profile of BPTS marker compounds was shown in Table 1. We expected the fragment pattern of marker compounds by reference to many of literatures. We confirm that the measured accurate ion masses and fragment patterns for standard and BPTS extract are almost identical. It means that expected marker compounds exist in BPTS. The elemental compositions of molecular and fragment ion were determined through comparing theoretical and measured mass of ions. When the standard mixture of marker compounds was analyzed six times to validate the repeatability for developed method, retention time and peak area were less than 1% and 5%, respectively.
[Table 1.] The chemical profile of BPTS marker compounds
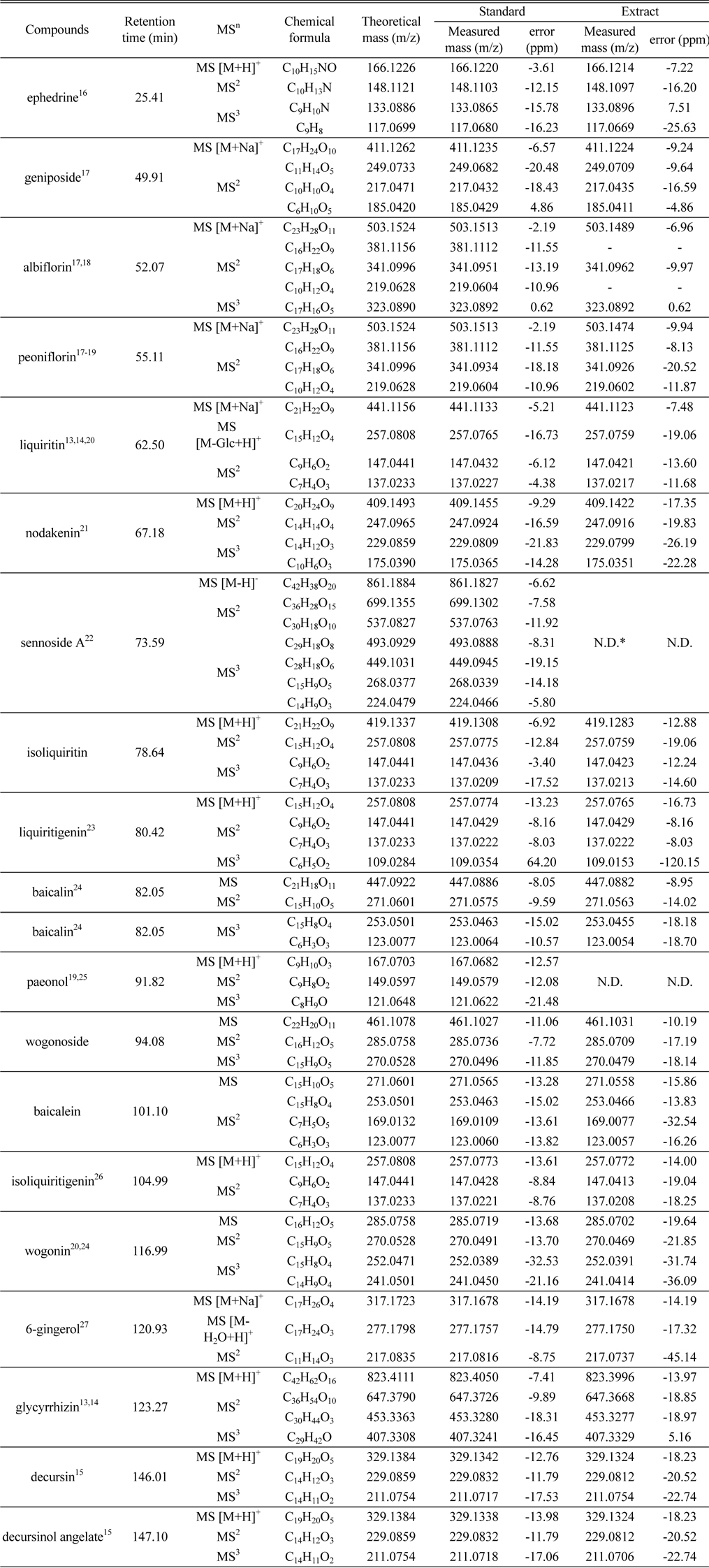
The chemical profile of BPTS marker compounds
A powerful and reliable analytical method using LC/UVIT- TOF mass spectrometry for qualification of 19 marker compounds in BPTS has been successfully developed. The accurate mass measurement capability and full spectral sensitivity of IT-TOF mass spectrometry enabled to identify multiple compounds and build chemical profile. The elemental composition and fragment pattern of marker compounds in BPTS extract were identified by comparing with standard reference. The mass errors of moelcular and fragment ions were almost under 20 ppm. The developed method was expected to make for quality control of BPTS.