In order to the feasibility of silk materials as protein delivery system, silk beads incorporated with bovine serum albumin (BSA) were prepared by dropping silk fibroin extract into dope solution composed of ethanol and dichloromethane. Structural and morphological characteristics of silk beads were examined using scanning electron microscopy (SEM), infrared spectrometry, and X-ray diffractometry. Swelling ratio of silk beads was also measured. Release behavior of prototypical protein, BSA, was studied by observing the electropheretic phenomenon and release profile. SEM showed that silk beads are spherical with porous interior structure. Infrared spectrometry and X-ray diffraction confirm that the silk beads have a β-sheet conformation. The swelling capability of silk beads increased with the incorporation of the protein. The protein was released from the beads with slow release following an initial burst release. Therefore, silk beads show promise as materials for encasing protein drugs to be delivered to targets in the human body.
Polymeric beads derived from natural sources are unique materials for controlled release due to inherent properties such as their non-toxic nature and biodegradation ability (Chavidi
Silk is a natural, fibrous protein spun by silkworms, and has been used traditionally in textile fibers and as surgical sutures for human subjects (Wang
In this study, we prepared silk beads and silk-BSA beads to examine the feasibility of silk beads as protein drug delivery systems. The morphological features of silk beads were observed using a scanning electron microscope. The structural characteristics of silk beads were examined using infrared spectrometer and X-ray diffractometer. The swelling ratio of silk beads was also measured. Electropheretic mobility was observed and release profile was evaluated to study the release behavior of model protein drug, BSA
>
Preparation of silk fibroin extracts
Silkworm cocoon was harvested from National Academy of Agricultural Science. The cocoon was degummed twice with 0.5% on-the weight-of-fiber (OWF) Marseilles soap and a 0.3% OWF sodium carbonate solution at 100℃ for 1 h and then washed with distilled water. Degummed silk fibroin fibers (35 g) were dissolved in a mixture (700 mL) of CaCl2, H2O, and ethanol (molar ratio 1:8:2) at 95℃ for 3 h. This calcium chloride/silk fibroin mixture was filtered twice through a Mira cloth (Calbiochem, San Diego, CA, USA) quick filter and dialyzed for three days to remove chemicals. The resultant SF solution was stored in the refrigerator before use (Kweon
Silk beads were prepared by dropping the SF solution into the dope solution, composed of dichloromethane and ethyl alcohol mixture solution (7:3 v/v). The mixture was stirred at 100 rpm and the SF solution (5%) was dropped by a peristaltic pump with a flow rate of 10 μL/s. The resultant mixture was then stirred for 2 h. The beads were collected after centrifugation (3000 rpm and 20 min), freezing overnight at −70℃, and then lyophilization. Table 1 shows the composition of the silk beads. BSA was used as a model drug.
[Table 1.] Preparation condition of silk beads

Preparation condition of silk beads
>
Scanning electron microscopy (SEM)
Bead morphology was characterized with a scanning electron microscope (JSM-11111). Beads were coated with platinum before mounting on carbon tape. The size of beads was measured by imaging through SEM and analysis using ImageJ tool.
Fourier transform infrared (FT-IR) absorbance spectra were obtained using an FT-IR spectrometer (Spectrum 100, Perkin Elmer, USA) in the spectral region of 2000 ~ 700 cm-1 at a resolution of 2 cm-1, and 32 repeated scans were averaged for each spectrum. X-ray diffraction (XRD) was performed by D8 ADVANCE with DAVINCI (Bruker, Germany) using Cu-Kα radiation. The X-ray source was operated at 40 kV and 40 mA.
The swelling ratio of beads immersed in water was measured as a function of time. The degree of swelling was calculated from the following equation:
Swelling ratio = (W2-W1)/W1,
where W1 is the original weight of the sample and W2 is the weight of dried residual sample after specific immersion time.
Beads (0.2 g) were dispersed into microtubes containing 200 μL of 1× phosphate-buffered saline (pH 7.4) and incubated at 37℃ in a horizontal orbital shaker, shaking at 50 rpm. At specific time points, beads were separated from the supernatant by centrifugation, and aliquots of the supernatant were taken for measurement. The buffer was replenished to maintain its original volume. The amount of released drug was calculated and expressed cumulatively. The amount of BSA released was measured using Bradford reagent (spectroscopic analysis) obtained from Sigma-Aldrich Korea (Yongin, Korea) with the absorbance measured at 562 nm. The calibration curves for all measurements were made with the test solution.
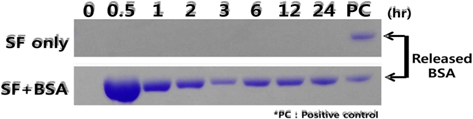
Release of BSA was also examined qualitatively using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE analysis was performed according to the Laemmli (1970) protocol followed by staining of the gel using Coomassie Blue R-250, and then washed thoroughly.
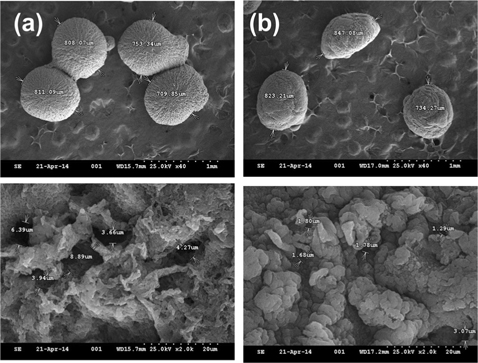
Silk and silk-BSA beads were prepared to examine their capability as a protein release matrix. The surface morphology of silk beads is shown in Fig. 1. As shown in Fig. 1(a), silk beads have a spherical, cavity-filled structure. The average bead size and pore length of silk beads are 770 μm and 5.4 μm, respectively. As shown in Fig. 1(b), the silk-BSA beads have an oval structure with randomly aggregated porous microstructure. The average bead size and pore length of silk-BSA beads are 800 μm and 1.64 μm, respectively. The microstructure of silk beads turned into an agglomerated, dense form with incorporation of BSA.
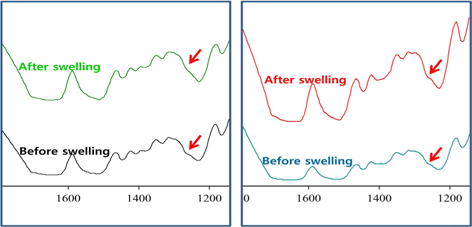
FT-IR is a powerful technique to examine the conformation of protein. The structural characteristics of silk beads and silk-BSA beads were analyzed using FTIR analysis. FTIR spectra of silk beads and silk-BSA beads are shown in Fig. 2(a) and Fig. 2(b), respectively. As shown in Fig. 2(a), SF beads exhibit a shoulder peak around 1265 cm-1, which is attributed to the β-sheet structure. SF-BSA beads (Fig. 2(b)) also show a similar peak around 1265 cm-1. IR spectra, representing typical absorption bands sensitive to the molecular conformation of silk fibroi, have been used by many researchers to determine the structure of silk fibroin. The absorption bands at 1660 (amide I), 1540 (amide II), and 1235 cm-1 (amide III), which were assigned to the random coil structure of silk fibroin, and 1630, 1530, and 1265 cm-1, assigned to β-sheet conformation. (Tsukada
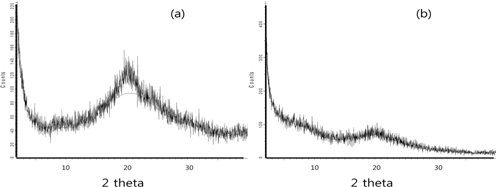
XRD analysis was performed to study the crystalline structure of silk beads and silk–BSA beads. XRD curves of the silk and silk-BSA beads are shown in Fig. 3. Silk beads (Fig. 3(a)) are characterized by the presence of strong diffraction peaks at Bragg angles (
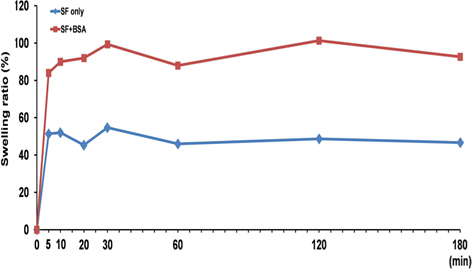
Swelling is an important factor affecting drug release from the matrix. In general, release ratio increased with an increase of swelling ratio. Therefore, we examined compared the swelling ratio of silk beads and silk-BSA beads (Fig. 4). Silk beads and silk-BSA beads were swellon rapidly within 5 min . Equilibrium swelling ratios of silk beads and silk-BSA beads were 50% and 95%, respectively. The silk-BSA beads have more swelling capacity than silk beads.
SDS-PAGE has been used to elucidate the release of model protein drug, BSA, from silk beads. Electrophoretic analysis (Fig. 5) shows that there is no stained region in the gel corresponding to the silk beads because of the absence of protein. The release pattern of BSA from silk-BSA beads was time dependent and sustained for 24 h. Burst release of BSA was observed in the electropheretic analysis within 0.5 h.
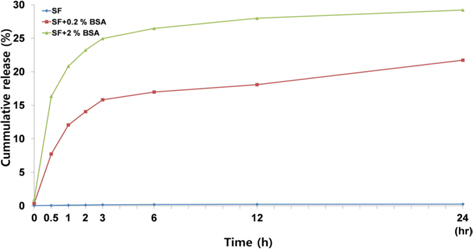
To elucidate the release profile of the BSA, we examined the release pattern with time. As shown in Fig. 6, BSA release could not be detected from silk beads. Most of the BSA was released from silk-BSA beads within 3 h, followed by a slow, continuous release. The cumulative release of BSA from silk-2% BSA beads and silk-0.2% BSA beads at 24 h was about 30% and 20%, respectively.
In our experiment, silk beads and silk-BSA beads were prepared by dropping SF extracts into an ethanol and dichloromethane mixture. The beads were spherical in shape and had a porous internal structure. The silk beads exhibited β-sheet conformation. The swelling ability of silk beads increased with the incorporation of a drug. The drug was released from the beads with initial burst release followed by continuous, slow release. It can be inferred from the above-discussed results that silk beads might be used as a drug release matrix for protein drugs.