Bees are critical for the pollination of natural vegetation and agricultural plants, including fruits, vegetables, seed plants, edible oil crops, garden flowers, and major forage crops. Pollination is, therefore, an ecosystem service, given that wild pollinators, particularly wild bees, contribute significantly to the pollination of a wide range of crops (Morandin and Winston, 2005; Greenleaf and Kremen, 2006; Winfree et al., 2007). Bees are both widely diverse and abundant, with 16,325 species currently identified worldwide (Michener, 2000). Commercially managed bees are available for pollination services and are used in large commercial fields, small gardens, and enclosures such as greenhouses and screen houses (Free, 1993; Dag and Kammer, 2001). The use of bumblebees for pollination in greenhouses has become widespread, and the demand for bumblebees increases every year. Bumblebees are particularly effective at pollinating species in the family Solanaceae, including tomato and eggplant (Buchmann and Hurley, 1978; Banda and Paxton, 1991; Free, 1993). It has been estimated that of the bumblebee colonies sold in 2004 approximately 930,000 were colonies of the Eurasian Bombus terrestris, approximately 55,000 were colonies of the North American B. impatiens , and a few thousand were colonies of the Eurasian B. lucorum , the East Asian B. ignitus , and the North American B. occidentalis (Velthuis and van Doorn, 2006).
Generally, Bombus species are annual, eusocial insects with short-lived colonies that are found primarily in temperate regions of the world. Queens are the only caste of Bombus to overwinter (enter diapause), whereas the workers and males perish in the late summer and early autumn, respectively. In the early spring, queens that have overwintered depart from their hibernation sites. The queen accumulates a store of pollen and then lays her first batch of eggs into the pollen mass after finding a suitable site for the foundation of a colony. Upon emergence, the workers of the first brood immediately assume the foraging activities of the queen, who from that point on spends most of her time laying eggs. In late summer, many males and new queens are produced. Only mated queens hibernate and emerge in the spring (Heinrich, 1979, Duchateau and Velthusis, 1988).
One of the key stages in the year-round rearing of B. ignitus bumblebees is the breaking of diapause. Diapause is defined as a stage in the development of certain animals during which morphological development may be suspended or markedly retarded (Andrewartha, 1952; Mansigh, 1971). Diapause most likely underlies the variation among queens that may lead to differences in the ability to produce offspring (Beekmand and Vanstratum, 2000). The programming of diapause involves the development of specific behavioral, morphological, and physiological design features that uniquely prepare the diapause-destined insect for a period of developmental arrest Denlinger, 2002). With the goal of inducing diapause break, several researchers have attempted to first induce hibernation in bumblebee queens under controlled conditions, despite their lengthy ovarian diapause Horber, 1961; Alford, 1969, 1975; Hoem, 1972; Beekman et al., 1998) and CO2 narcosis (Röseler and Röseler, 1984; van den Eijnde et al., 1991). Under natural conditions, the duration of the hibernation period ranges from 6 to 9 mo (Alford, 1969a). Hoem (1972) maintained hibernating B. terrestris queens in mounds of soil in unheated greenhouses or in plastic containers with perlite as bedding; the bees were subsequently placed into a refrigerator at 4-5℃ for 8-9 mo. Asada (2004) reported that chilling in a refrigerator at 5℃ for 4 mo was effective procedure for inducing nest initiation by B. h. hypocrite queens. Diapausing bumblebee queens require sizeable fat reserves, which are used up during the diapause period, and the amount of metabolic reserves remaining after diapause completion depends primarily on diapause length (Hoem, 1972). Beekman et al. (1998) demonstrated that the weight of bumblebee queens prior to entering diapause affects their survival during diapause; queens with a wet weight below 0.6 g were incapable of surviving diapause, regardless of diapause length. Although increased survival rates have been reported in some studies, few studies have attempted to evaluate the effects of different diapause conditions, including chilling temperature, humidity, duration, and timing, on the diapause survival rates of queens and their subsequent ability to establish a colony.
To evaluate the effects of the timing and duration of chilling on diapause break in B. ignitus queens, we determined whether the timing of the application of cold temperatures and the duration of chilling affect the artificial hibernation of B. ignitus queens. In this study, we determine and describe the most favorable timing and duration of cold temperature application for breaking diapause in B. ignitus queens.
The insects used in the experiment were second and third generation queens acquired from B. ignitus colonies that were reared year-round in a climate-controlled room (27℃, 65% relative humidity, and continuous darkness) at the Division of Applied Entomology, Department of Agricultural Biology, National Academy of Agricultural Science, Republic of Korea.
The basic colony-rearing technique used in the present study was described previously by Yoon et al. (2002). The queens were reared in three different types of plastic boxes for nest initiation (10.5 × 14.5 × 6.5 cm), colony foundation (21.0 × 21.0 × 15.0 cm), and colony maturation (24.0 × 27.0 × 18.0 cm). Queens were first confined individually in small boxes for colony initiation and remained there until oviposition. After the adults emerged from the first brood, the nest was transferred to a medium box for colony foundation and was stored there until the number of workers reached 50. The nest was subsequently moved to a large box for further colony development. A 40% sugar solution and pollen dough were provided ad libitum. The pollen dough was composed of a sugar solution and pollen (v:v 1:1).
To evaluate the effects of timing of cold temperature application on diapause break in B. ignitus queens, the following environmental conditions were established. The cold time regimes were set as 12 d, 40 d, and 100 d after emergence under a constant temperature of 5℃ and 80% humidity. After mating (Yoon et al., 2008), the mated B. ignitus queens were weighed prior to the initiation of the experiment. The queens were individually preserved in bottles filled with perlite in a perforated plastic box containing perlite to prevent mold growth and stored for 5 mo in a different chilling chamber (in continuous darkness). The survival rate of queens was surveyed each month. Thirty queens were used in this experiment. To obtain a more precise picture of the effects of the timing of cold temperature application on B. ignitus queens, additional cold time regimes were set as 8 d, 12 d and 16 d after emergence under a constant temperature of 0℃, 2.5℃ or 5℃ and 80% humidity. Thirty queens and 3 replications were used in this experiment.
To determine the chilling duration that was most favorable for artificial hibernation in B. ignitus queens, cold periods ranging from one month to four mo under a constant temperature of 5℃ and 80% humidity were tested. B. ignitus queens that had been chilled for 15 d were used as a control in the chilling duration experiment. Mated B. ignitus queens (12 d after emergence) were weighed and stored in a perlite-filled bottle in a perforated plastic box containing perlite in a 5℃ chilling chamber for 1 to 4 mo. Thirty B. ignitus queens were employed in each cold period tested in this experiment. At the end of treatment, the surviving queens were again weighed. The queens were then transferred to flight cages for three d (Yoon et al., 2004b). Each queen was reared in a climate-controlled room (27±1℃, 65% R.H. and continuous darkness). The developmental ability of each colony was estimated based on the rates of oviposition, colony foundation, and progeny-queen production. The queens that did not evidence oviposition within 40 d were excluded from the analysis of the number of oviposited colonies (Yoon et al., 2004a). Colony foundation was defined as the time period necessary for more than 50 workers to emerge from a colony.
Statistical analyses were conducted using Chi-square tests (MINITAB Release 13 for Windows, 2000).
Optimal timing of cold application for artificial hibernation of B. ignitus queens
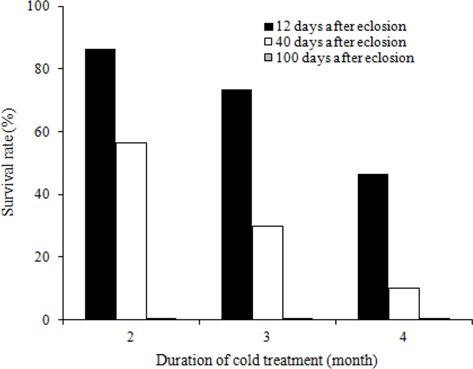
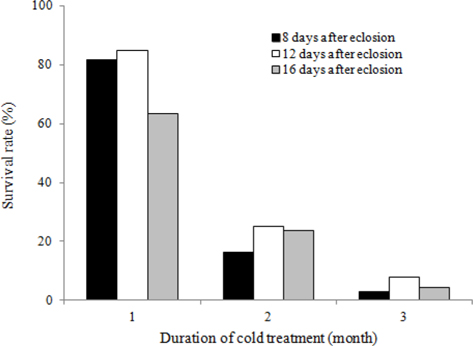
To evaluate the effects of timing of cold application on diapause break in B. ignitus queens, we evaluated the survival rates of queens stored at different d after emergence under a constant temperature of 5℃ and 80% humidity (Fig. 1). Of the queens stored at 12 d, 40 d, and 100 d after emergence, the queens stored at 12 d after emergence evidenced the highest survival rates, which were 86.7% at two mo, 73.3% at three mo, and 46.4% at 4 mo. Survival rates were reduced at the following timing: 12 d, 40 d, and 100 d after emergence. The survival rates of the B. ignitus queens were affected by the timing of cold application (Chi-square tests: x2=46.581, df=2, p=0.0001 at 2 mo; x2=36.118, df=2, p=0.0001 at 3 mo; and x2=23.642, df=2, p=0.0001 at 4 mo). To obtain a more precise picture of the effects of the timing of cold temperature application on B. ignitus queens, additional cold time regimes were set as 8 d, 12 d and 16 d after emergence under a constant 80% humidity (Fig. 2). Of the queens in cold treatments applied at 8 d, 12 d, and 16 d after eclosion, the queens stored at 12 d after eclosion exhibited the highest survival rates, which were 84.6 at one mo, 25.0% at 2 mo, and 7.9% at 3 mo. There were significant differences between the evaluated times of cold application at one mo after cold treatment initiation (x2=13.629, df=2, p=0.001 at one mo; x2=2.370, df=2, p=0.306 at 2 mo; and x2=6.583, df=2, p=0.037 at 3 mo).
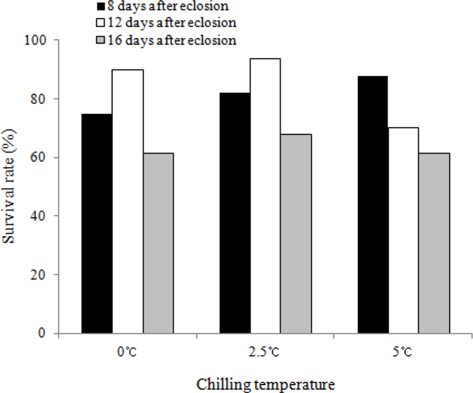
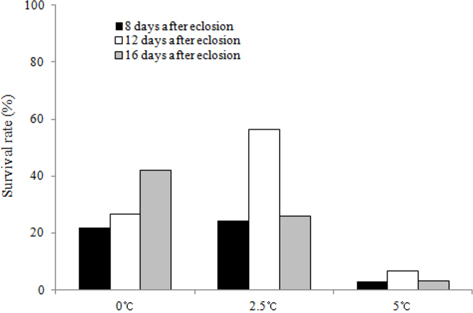
We investigated the survival rates of B. ignitus queens subjected to chilling treatments at different times (8, 12, and 16 d after emergence) and temperatures (0℃, 2.5℃ and 5℃). The queens stored at 12 d after eclosion and 2.5℃ exhibited the highest survival rates, which were 93.5% at one mo (Fig. 3) and 56.3% at 2 mo (Fig. 4). Lower survival rates were observed at 2.5℃, 0℃, and 5℃. The survival rates of the B. ignitus queens were affected by chilling time and temperature (Chi-square tests: x2=21.053, df=8, p=0.007 at 1 mo and x2=36.984, df=8, p=0.0001 at 2 mo). Therefore, our results demonstrate that 12 d after emergence is the most favorable time for application of cold treatment for the break of diapause in B. ignitus. Yoon et al. (2010) reported that optimal timing for cold application in B. terrestris is 8 d after emergence.
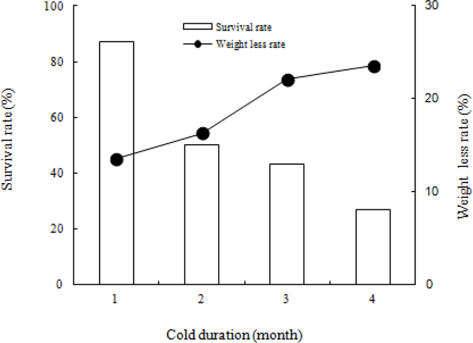
We investigated the survival rate and rate of weight loss in B. ignitus queens at different lengths of time under a constant 5℃ and 80% humidity (Fig. 5). A shorter cold period appeared to correspond to a higher survival rate: 87% at 1 mo, 50% at 2 mo, 43% at 3 mo, and 27% at 4 mo. The survival rate of B. ignitus queens was significantly affected by chilling period (Chi-square test: x2=23.092, df=3, p=0.0001). Fig. 5 shows the changes in the weights of B. ignitus queens after artificial hibernation at different cold periods. The rates of weight loss after artificial hibernation over the course of 1, 2, 3, and 4 mo were 13.5%, 16.3%, 22.1%, and 23.6%, respectively. The rate of weight loss appeared to increase as chilling durations grew longer. The rates of weight loss in B. terrestris queens after chilling treatment durations of 3 and 5 mo were 9.0-14.0% and 21.3-30.9%, respectively (Yoon et al., 2010). Horber (1961) previously determined that the average weight loss during hibernation was 151.3 mg. Furthermore, the same study demonstrated that surviving queens were heavier than the queens that died during hibernation. Hoem (1972) reported that the observed weight loss occurred during the first half of the hibernation period and that body weight increased after that point. Significant positive correlations were found between the body weight of the queens and the length of survival during and after hibernation. The weight of B. terrestris queens ranged from 400-1000 mg prior to hibernation and from 300-900 mg after hibernation. Similarly, the weight of B. lapidarius queens ranged from 250-850 mg prior to hibernation and from 250-750 mg after hibernation.
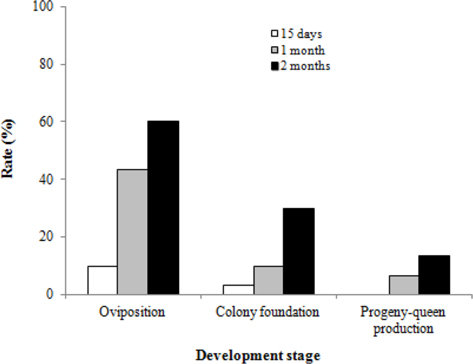
We surveyed the colony developmental characteristics of B. ignitus queens subjected to chilling for different lengths of time. The rates of oviposition, colony foundation, and progeny-queen production of queens that hibernated for 2 mo were 60.0%, 30.0% and 13.3%, respectively (Fig. 6). These values were 6.0 to 13.3 times higher than those of the queens that hibernated for 15 d. The rate of oviposition in B. ignitus queens reared after diapause was affected significantly by the chilling period during diapause (Chi-square test: x2=16.544, df=2, p=0.0001). However, there were no significant differences in the rate of colony foundation or progeny-queen foundation (x2=4.275, df=2, p=0.118 for colony foundation rate and x2=4.286, df=2, p=0.117 for progenyqueen foundation). In view of this survival rate and the colony developmental characteristics after artificial hibernation, the most favorable cold period was determined to be at least 2 mo.
The results of the present study demonstrate the most favorable timing of cold application and the optimal chilling duration to induce diapause break in B. ignitus queens. Collectively, the findings of the present study indicate that application of cold treatment at 8 d after emergence and a cold period of at least 2 mo were the most favorable conditions for diapause break in B. ignitus queens.