This study was designed to find out the effect of Nosema spore on oxidative damages and antioxidant defence in the midgut of tasar silkworm Antheraea mylitta. Higher level of lipid peroxidation (LPX) and total hydroperoxides indicate the resultant oxidative stress in the Nosema exposed specimen. Increased superoxide dismutase (SOD) suggests activation of physiological mechanism to scavenge the superoxide radical produced during Nosema infection. Higher activities of catalase and glutathione-S-tranferase on 18th d indicate adaptive behaviour of the tissue against oxyradicals. The results suggest that Nosema infection is involved in altering the active oxygen metabolism by modulating LPX and reactive oxygen species (ROS), which is indicative of pebrine disease disorder.
Tropical tasar silkworm,
To protect against these toxic cellular environment, insect possess a suit of antioxidant defence mechanisms, comprised of both enzymatic as well as non-enzymatic components. The major enzymes involved in the process are superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione reductase (GR) and glutathione-S-transferase (GST). Superoxide radicals (O2•‾) are dismutated by SOD to hydrogen peroxide (H2O2) which is reduced to water and molecular oxygen by CAT. Further, GPX catalyzes the reduction of H2O2 to water and organic peroxide to alcohols using reduced glutathione GSH as a source of reducing equivalent. GR regenerates reduced glutathione (GSH) from oxidized glutathione (GSSG), which is a scavenger of ROS as well as a substrate for the other enzymes. GST conjugates xenobiotics with GSH for excretion. The non-enzymatic component consists of small organic molecules such as GSH, and vitamin C (Felton and Summers,1995; Krishnan and Kodrik,2006; Krishnan
Sufficiently large details are available on larval growth rate (Rath
>
Isolation of spores and Insect infection
The midguts were dissected out, thoroughly washed in ice-chilled phosphate buffer (50 mM, pH 7.0) to remove haemolymph, muscle tissues and fat body contamination. Tissue samples (midguts) were homogenized in ice-cold buffer (50 mM phosphate buffer, pH 7.0). Homogenization was carried out in an ice-chilled motor driven Teflon Potter-Elvejhen homogenizer and centrifuged at 8000 x g for 15 min at 4℃. The supernatant was used for biochemical analysis.
>
Estimation of lipid peroxidation
LPX level was assayed by measurement of malondialdehyde (MDA), a decomposition product of polyunsaturated fatty acids hydro peroxides were determined by the TBA reaction as described by Bar-Or
>
Estimation of total hydroperoxides
Total hydroperoxides were determined spectrophotometrically according to the method of ferrous oxidation with xylenol orange (FOX1) (Wolff, 1994). Hydroperoxides oxidize ferrous to ferric ions selectively in dilute acid and the resultant ferric ions can be determined by using ferric sensitive dyes as an indirect measure of hydroperoxide concentration. Xylenol orange binds ferric ions with high selectivity to produce a coloured (blue-purple) complex. The absorbance was read at 560 nm after removal of any flocculated material by centrifugation at 4000 x g for 10 min. The signal was read against an H2O2 standard curve.
Super oxide dismutase activity was estimated by Kono, (1978). The reaction mixture consisted of 50 mM sodium carbonate, 25 μM NBT, 0.6% Triton X 100 and 0.1 mM EDTA. The reaction was initiated by addition of 1 mM hydroxylamine-hydrochloride. The rate of NBT reduction was recorded at 560 nm. The control was simultaneously run without tissue homogenate. One unit of SOD is defined as the amount required inhibiting the photoreduction of NBT by 50%. The specific activity of SOD was expressed as unit/mg protein.
Catalase activity was determined according to Aebi, (1974). The method is based on the decomposition rate of H2O2 by the enzyme. The assay mixture contained 2.9 mL of 12 mM H2O2 and 0.1 mL of sample (100 μg protein). Absorbance was measured at 240 nm and CAT activity is expressed as nkat/ mg protein (1 katal = 1 mol sec-1).
Glutathione-S-transferase activity was measured according to Habig
The protein content was estimated by the Bradford (1976) method using bovine serum albumin as standard.
Results were expressed as mean ± standard deviation (SD). Difference between control and treatment was analyzed by Student’s t-test. Differences were considered statistically significant when
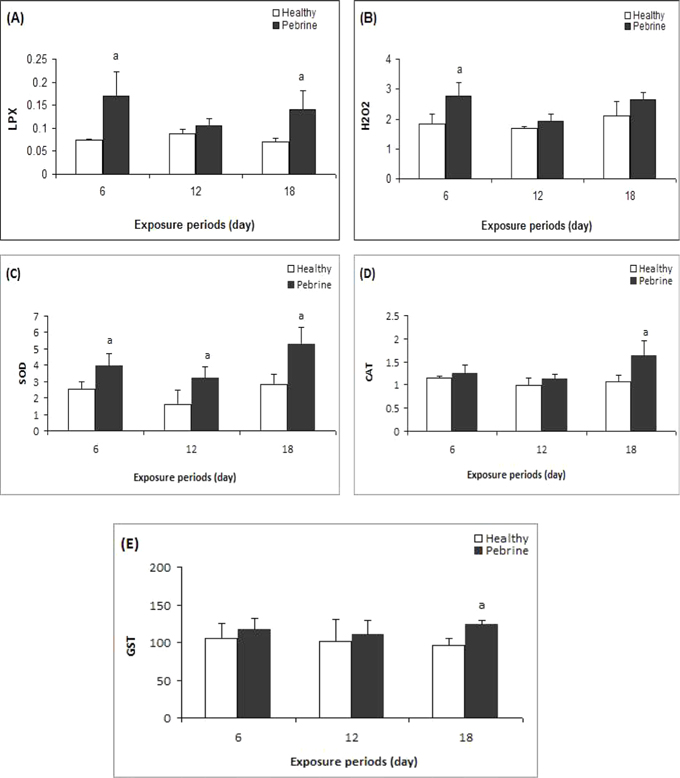
A significant increase in LPX level was observed in the midgut of
Total hydroperoxide level significantly increased on 6th day of treatment (Fig-1B,
A significant increase in SOD activity to was seen up to (1.55 fold) on the 6th day, 1.98 fold on 12th day and 1.89 fold on the 18th day in the larvae exposed to
CAT activity showed a significant increase on 18th day of exposure compared to control larvae (Fig-1D,
GST activity in the midgut of infected larvae significantly increased on 18th day after inoculation in comparison to the control (Fig-1E,
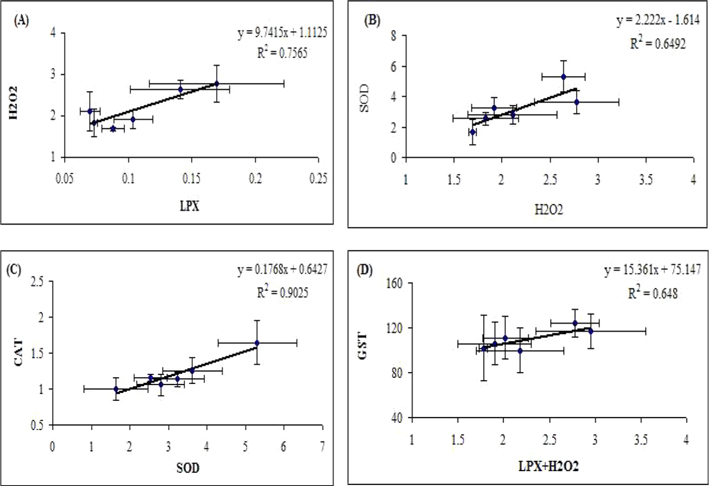
LPX serves as an indicator of oxidative damage in cells and tissues (Pampanin
Determination of antioxidant status of larva exposed to
The principal H2O2 scavenging enzyme CAT showed increased level in midguts of
GSTs are involved in the detoxification of both reactive intermediates and oxygen radicals (Van der Oost
The present study demonstrates that