Caffeine is a thermogenic agent that can be used in weight loss products. In order to achieve a sustained release of caffeine, silk fibroin (SF) film was uses as carrier. It has been shown that the loading method of caffeine into SF film affected the uniform distribution of caffeine in the SF film. When caffeine was added directly into SF solution, gelation has been occurred immediately and prevented the uniform distribution of caffeine. On the other hand, caffeine was dissolved in methanol in order to load the caffeine in SF film and crystallize the SF film at the same time. However, due to the fast evaporation of methanol, caffeine was recrystallized on the surface of SF film rather than penetrating into the film. Finally, caffeine was loaded into pre-crystallized SF film and uniform distribution of caffeine could be achieved. There was an initial burst of caffeine during the first 15 min, but after that a sustained release was achieved.
Obesity is one of the threats to the health of human and its control is important not only for personal health care but also for reducing social health care costs. The obesity is a result of excess energy intake over energy expenditure. Therefore, any substances which can increase the energy expenditure such as caffeine, ephedrine, capsaicin, and green tea can be used for obesity management (Diepvens
Silk fibroin (SF) has been used as a drug carrier for various drugs. The advantage of SF in drug delivery is that the entrapment of drug did not need any chemical cross-linking. SF became insoluble by various methods including exposure to alcohols or moisture. These methods promote the formation of β-sheet structure which acts as a physical cross-link, and thereby the activity loss of drug could be minimized.
The aim of this study is to develop a loading strategy of caffeine in the SF film. Three different loading strategies were applied in this study. First, caffeine was dissolved in SF aqueous solution and casted into film. Second, SF film was crystallized with caffeine dissolved methanol. Finally, caffeine was loaded after crystallizing the SF film with methanol. This is a preliminary study that suggests an effective loading strategy based on the uniform distribution of caffeine in the film.
Silk cocoon was obtained from National Academy of Agricultural Sciences. All other chemicals were purchased from Sigma-Aldrich LTD. (Yongin, Korea).
>
Preparation of SF aqueous solution
Cocoon were cut into 8 equivalent pieces, and immersed into the degumming solution consist of 0.3 wt% of sodium oleate and 0.2 wt% of sodium carbonate. The degumming was performed at 100°C for 1 h, and the final degumming rate was 28.1%. The degummed SF fibers were dried in an oven at 50°C for 2 d. The dissolution of SF fibers (40 g) was carried out with 200 mL of 9.3 M LiBr solution at 50°C for 4 h. The SF solutions went through dialysis in a dialysis tube (Spectra/Por®, MWCO 6-8,000, USA) against distilled water for 72 h. The dialysis water was changed every 2 h during the day time. The final concentration of the aqueous SF solution was 5.6% (w/v), but adjusted by adding distilled water to the solution if required.
>
Loading of caffeine into SF film
Three different loading methods were applied to load caffeine in the SF film. First, 0.45 g of caffeine was dissolved directly in 30 ml of 4% (w/v) aqueous SF solution. The caffeine dissolved SF solution was casted on a polypropylene weighing dish at 80°C. This caffeine-loaded SF film will be designated as Cafd- SF. Second, pure SF solution was casted into film on the same weighing dish at 80°C prior to the caffeine loading. The crystallization of SF film was performed with methanol containing 0.9% (w/v) caffeine. The methanol was evaporated in the fume hood at room temperature. This caffeine-loaded SF film will be designated as Caf-m-SF. At last, pure SF film was prepared by casting the solution at 80°C and crystallized with pure methanol. The loading of caffeine was performed by immersion of the film in 2% (w/v) aqueous caffeine solution for 5 h at room temperature, and dried in the fume hood at room temperature. This film will be designated as Caf-p-SF.
>
Evaluation and analysis of caffeine-loaded SF film
The appearance of the caffeine-loaded SF film was evaluated by the optical images and field emission scanning electron microscope (FE-SEM, JSM-7600F, JEOL, Japan) images of the prepared film. Attenuated total reflection-Fourier transformed infrared spectrometer (ATR-FTIR, MIDAC, Japan) was used to analyze the chemical structure of loaded caffeine and the secondary structure of SF. The released amount of caffeine from the film was measured using UV-Vis spectrometer (Optizen 2120UV, Mecasys, Korea). The Caf-p-SF film were cut into 1cm x 1 cm and immersed into 10 mL of 10 mM sodium phosphate buffer (pH 7.4). One milliliter of solution was sampled from the test solution in every 15 min, and the same volume was refilled with fresh buffer solution. The absorbance at 274 nm was monitored, and there was no interference by SF.
In order to ensure the uniform release of caffeine, the loaded caffeine should be distributed in the SF film uniformly. The penetration of caffeine through the dermal layer would be governed by the concentration gradient developed at any contact point between caffeine-loaded film and skin. If the concentration gradient is not uniform throughout the film, then the caffeine delivery would not be uniform or unpredictable. Therefore, the primary selection rule was the uniform distribution of caffeine in the film.
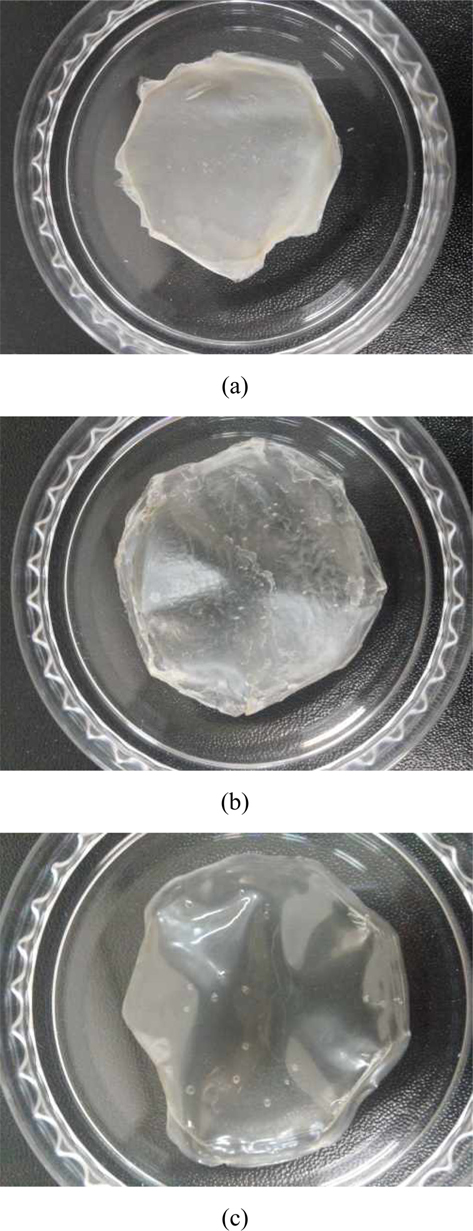
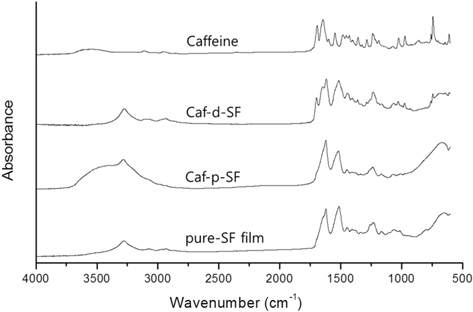
First, we dissolved caffeine directly into SF solution and tried to cast into a film (Caf-d-SF). This method will ensure the uniform distribution of caffeine in SF film, and it is most widely used for drug loading in SF film. However, upon the addition of caffeine, the SF solution went gelation immediately, which prevented the formation of uniform film (Fig. 1a). In addition, the gelation also prevented the uniform dissolution of caffeine in the SF solution. The gelation of SF might be promoted by the presence of two carbonyl bond of the caffeine which could act as a hydrogen acceptor. The ATR-FTIR spectrum of Cafg- SF shows that the secondary structure of SF turned into β-sheet structure (1620 cm-1) without the methanol treatment. Previously, in the same casting condition, SF film had random coil structure (Lee, 2004). Caffeine might act as a physical crosslinker which bring SF molecules into close distance and facilitate the formation of β-sheet structure. Furthermore, the peaks of caffeine could be also observed, which corresponds to the strong bands at 1700 and 1655 cm-1. that assigned to C=O asymmetric and symmetric stretching, respectively (Liedana
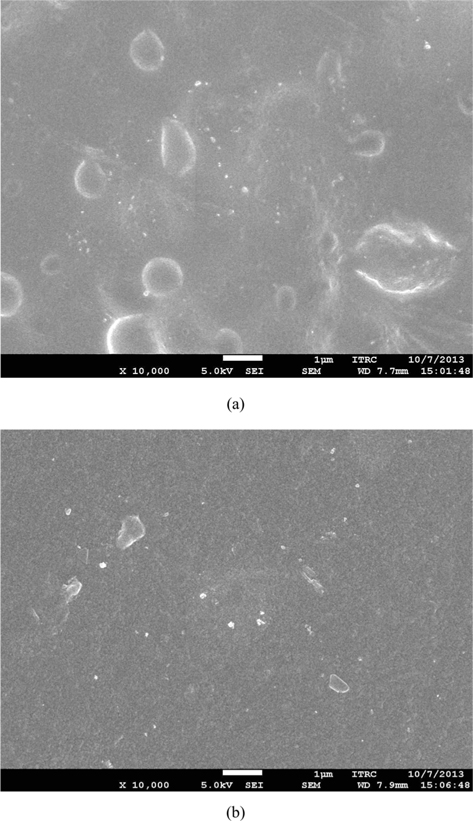
Above result indicates that the direct dissolution of caffeine in SF solution is not recommendable for caffeine loading. As an alternative, we tried to load the caffeine during the crystallization of SF film with methanol (Caf-m-SF). Caffeine was dissolved in methanol and used for the crystallization of SF film. However, this method was also found to be not adequate for uniform loading of caffeine. Recrystallization of caffeine was observed in both macroscopical (Fig. 1b) and microscopical (Fig. 2a) level. The recrystallizaton might be occurred due to the limited solubility of caffeine in methanol. The solubility of caffeine in methanol is only 0.048 M or 0.92% (w/v) (ONS Challenge Lab Notebook Page Website). The experiment was designed to have maximum concentration gradient in order to facilitate the penetration of caffeine into the SF film. Therefore, caffeine was dissolved in methanol almost to the saturation level. However, the methanol evaporated too fast then expected, which resulted in recrystallization of caffeine prior to penetration into the SF film.
In the last method, SF film was crystallized by methanol before the caffeine loading (Caf-p-SF). The methanol treatment makes the SF film insoluble, but it does not mean that the film cannot be swollen. We expected that the caffeine could be penetrated into the amorphous region of SF film during swelling. The optical image of Caf-p-SF film shows that the film is transparent, which indicates the uniform distribution of caffeine (Fig. 1c). The FE-SEM image of Caf-p-SF also shows a smooth surface with little defects (Fig. 2b). The ATR-FTIR also revealed the uniform distribution of caffeine (Fig. 3). Unlike to Caf-d-SF, the peaks of caffeine could not be observed anymore, which indicates caffeine crystals were not formed even though caffeine was dissolved in the buffer near to the saturation level. Compared to the pure SF film, the peak intensity of Caf-p-SF film at amide I (1700-1600 cm-1) was increased. It might be due to the incorporation of caffeine because caffeine has also a strong peak in the same region. The peak of near 3500 cm-1 in the Cafp- SF film was also increased compared to the pure SF film due to the water that remained even after drying. The same could be observed in the PVA film (Li
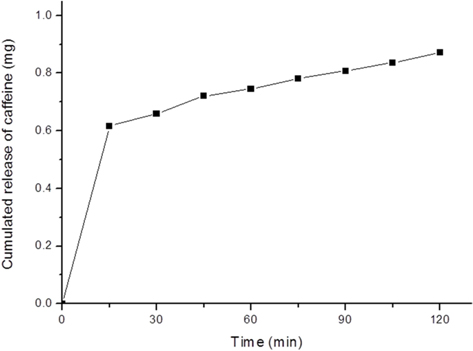
The release profile of caffeine from the Caf-p-SF film is shown in Fig. 4. There was an initial burst of caffeine during the first 15 min but after that a sustained release were achieved. Our results indicate that the loading method can affect the uniform distribution of caffeine in the SF film. We expect that the caffeine-loaded SF film could be used for weight loss patches.