In the present study, we report that the selection of operation mode is important to take the full advantage of nanofibrous membrane in enzyme immobilization. Silk fibroin nanofibrous membrane has been prepared by electrospinning, and α-chymotrypsin was immobilized as a model enzyme. When the immobilized enzyme was operated in the membrane reactor mode, the Michaelis constant, Km, was lower and the Vmax was higher compared to the batch reactor mode. No concentration gradient was observed in the membrane reactor mode and the immobilized enzyme was stable even after 7 times of re-use. Our results suggests that the enzyme immobilized nanofibrous membrane should be operated in the membrane reactor mode rather than in the bath reactor mode
Enzymes have advantages over chemical catalysts because of regio- and site-specificity (Koeller
A nanofibrous membrane prepared by electrospinning has been used in various fields due to its high specific surface area, and it could be an excellent candidate for the enzyme immobilization. There are many studies on the enzyme immobilization onto nanofibrous membrane (Wang
In the present study, we immobilized CT onto electropspun SF nanofibrous membrane and operated in two different systems. First, it was operated like a batch-type reactor, where the enzyme immobilized SF nanofibrous membrane was immersed into the substrate solution. Secondly, the enzyme immobilized SF nanofibrous membrane was mounted on the membrane holder and the substrate solution was passed through the membrane. We compared the Michaelis-Menten parameters of both operations and examined the effectiveness of the membrane reactor.
Silk cocoon was obtained from Hung Jing Co., LTD. (Seoul, Korea). All other chemicals including the CT were purchased from Sigma-Aldrich LTD. (Yongin, Korea).
>
Preparation of Regenerated SF
Regenerated SF was prepared by following procedure. Silk cocoons were degummed twice with 0.3% (w/v) Marseilles soap and 0.2% (w/v) sodium carbonate solution at 100ºC for 1 h, and rinsed thoroughly with warm distilled water. Degummed silk cocoons were first dissolved in a ternary solvent system of CaCl2/ H2O/EtOH solution (1/8/2 mole ratio) for 30 min at 85ºC. Aqueous SF solution is obtained by dialysis of dissolved SF solution in a cellulose tube (molecular weight cutoff = 12,000-14,000) against distilled water for 4 d at room temperature. Then, aqueous SF solution was freeze-dried to obtain regenerated SF sponge.
>
Preparation of SF nanofibrous membrane
The regenerated SF sponge was dissolved in formic acid (98- 100%) to achieve final concentration of 12% (w/v). The silkformic acid solution was placed in a 3-mL syringe (G-22). The tip-to-collection plate distance was 10 cm and, a voltage of 12 kV was applied to the collection drum. The electrospun SF nanofibers were collected on an aluminum foil, which covers the collection drum, in a form of nonwoven webs. After spinning, it was removed from the foil and immersed in methanol (99%) for 1 h. Finally, it was dried under tension to prevent shrinkage and cut into round shape to fit the filter holder (2.5 cm i.d.).
>
Immobilization of CT on SF nanofibrous membrane
In order to immobilize CT on the SF, the amine groups of SF were activated with glutaraldehye (GA). To one piece of SF nanofibrous membrane, 10% (v/v) GA in 0.2 M sodium carbonate buffer (pH 9.2) was added to activate the SF. The reaction was continued for 1 h at 25ºC. The activated SF was washed 2 times with distilled water and 3 times with 0.1 M sodium phosphate buffers (pH 7.4). The activated SF was mounted on the membrane holder and an ice-cold buffer solution of CT (1 mg/mL) in 0.1 M sodium phosphate buffer (pH 7.4) was circulated overnight at 4ºC. The unbound CT was washed out 20 mL of ice-cold 0.5 M of NaCl in 0.1 M sodium phosphate buffer (pH 7.4). Further it was washed 30 mL of the same buffer but without NaCl.
>
Quantification and Activity Test of CT
The bound CT was measured by bicinchoninic acid assay methods using bovine serum albumin as standard. The amount of bound CT was calculated by subtracting the amount of remaining CT from the initial amount of CT. N-benzoyl-DLtyrosine- p-nitroanilide hydrochloride (BTPNA) was used as substrate of CT. For general purpose, the substrate solution were prepared by mixing 5 mM BTPNA in DMSO, distilled water and 0.1 M sodium phosphate buffer (pH 7.4) in a ratio of 10:15:3, respectively. In order to measure the activity of the immobilized CT, the increase of absorbance at 400 nm was measured using UV spectrometer (UVICON 923, Kontron Instruments, USA). If the value of absorbance exceeded 2 then it was diluted until it reached below 1.5. The activity of immobilized CT was defined as μmol BTPNA hydrolyzed in 1 min by 1 mg of immobilized CT applying an absorbance coefficient of ε400 = 9520 M-1cm-1. The Michaelis-Menten parameters was calculated by the Lineweaver–Burk plot after reacting enzyme immobilized SF nanofibrous membrane with the substrate solution having different BTPNA concentration. In this case, the final concentration of BTPNA was 0.0625, 0.125, 0.25, 0.5, 1.0 and 2.0 mM.
>
Operation modes of immobilized CT
The substrate solution was reacted with enzyme immobilized SF nanofibrous membrane in two modes. For the batch reactor mode, the enzyme immobilized SF nanofibrous membrane was immersed in 10 mL of substrate solution and incubated for 30 min at 25ºC. For the membrane reactor mode, the enzyme immobilized SF nanofibrous membrane was mounted on the membrane holder, and the substrate solution was passed through the membrane using a peristaltic pump just once at 25ºC. The flow rate of the substrate solution was changed in order to optimize the reaction condition for membrane reactor mode. For the recycling of immobilized CT, 10 mL of substrate solution was passed through the membrane in each cycle, and the membrane was washed with 30 mL of 0.1 M sodium phosphate buffer (pH 7.4) during the intervals.
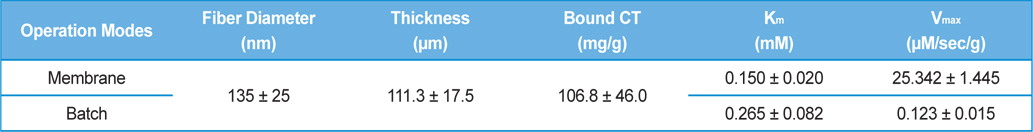
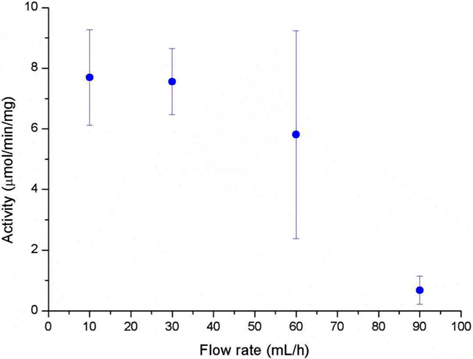
A high surface area and low diffusional limitation is the fundamental requirements in the selection of morphology of support for enzyme immobilization. High surface area enables high enzyme loading which can compensate the loss of activity after immobilization. Low diffusional limitation is required in order to maximize the enzyme activity. Porous materials are favored due to the high surface area but the diffusional limitation is usually accompanied. Non-porous materials can be free from the diffusional limitation but low surface area would be more problematic. From this point of view, an electrospun nanofibrous membrane has a high surface area due to its fine diameter, and each fiber is considered as non-porous material. The spaces between the fibers could be considered as pores but they are far greater than that could be found in a porous material. In this study, we prepared SF nanofibrous membrane by electrospinning. The mean diameter of SF nanofiber was 135 ± 25 nm and the thickness of the SF nanofibrous membrane was 111.3 ± 17.5 μm (Table 1). The amount of CT that immobilized onto 1 g of SF was 106.8 ± 9.6 mg. Since the flow rate of substrate solution through the membrane defines the retention time of enzyme in the membrane, we first checked the effect of flow rate on the activity of immobilized CT. As shown in Fig. 1, the activity of immobilized CT decreased with the increase of flow rate. It is obvious that the activity of immobilized CT will be increased with prolonged retention time. Based on the results of Fig. 1, the flow rate was fixed to 30 mL/h for further experiments. In our previous reports, the enzyme immobilized SF nanofibrous membrane was operated like a batch type reactor (Lee
[Table 1.] Dimension of SF nanofibrous membrane and kinetic parameters of immobilized CT
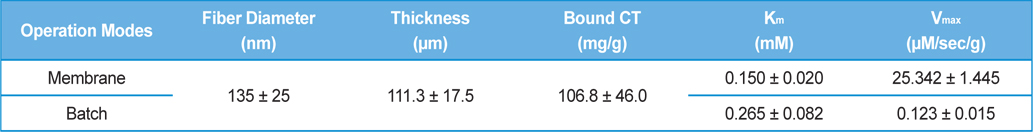
Dimension of SF nanofibrous membrane and kinetic parameters of immobilized CT
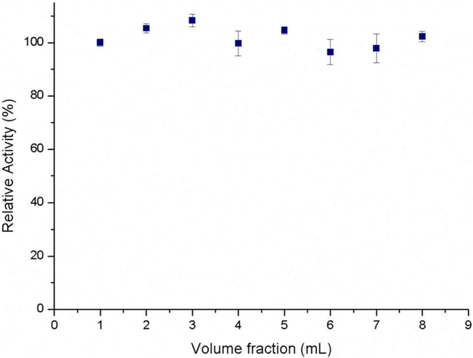
Fig. 2 shows the relative absorbance of each volume fraction that eluted from the reactor. The absorbance is maintained throughout the volume fraction indicating that there is no void volume at the beginning. In the case of packed-bed reactor, there is concentration gradient at the beginning of reaction and needs to be stabilized (Balcão
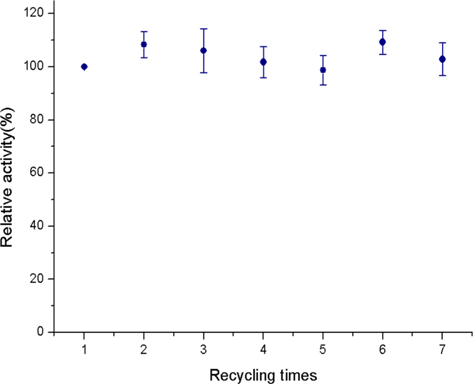
Fig. 3 shows the operational stability of the immobilized CT. Even after 7 times of re-use, the activity of the immobilized CT was maintained. The operational stability of an immobilized enzyme is the most important factor that determines the potential of the system for industrialization. In the membrane operating mode, high pressure was applied in order to pass the substrate solution through the membrane. Our concern was whether such high pressure can denature the immobilized enzyme. However, our result indicates that the CT immobilized onto the SF nanofibrous membrane did not affected by the pressure and have high potential for industrialization.