The oil in mackerel muscle was extracted using an environment friendly solvent, supercritical carbon dioxide (SC-CO2) at a semibatch flow extraction process and an n-hexane. The SC-CO2 was maintained at a temperature of 45℃ under pressures ranging from 15 to 25 MPa. The flow rate of CO2 (27 g/min) was constant during the entire 2 h extraction period. The fatty acid composition of the oil was analyzed using gas chromatography (GC). Significant concentrations of eicosapentaenoic acid (EPA) acid and docosahexaenoic acid (DHA) acid were present in the SC-CO2 extracted oil. The oil extracted using SC-CO2 exhibited increased stability compared with n-haxane extracted oil. Particles of mackerel oil together with the biodegradable polymer, polyethylene glycol (PEG) were formed using a gas saturated solution process (PGSS) with SC-CO2 in a thermostatted stirred vessel. Different temperatures (45-55℃), pressures (15-25 MPa) and a nozzle size 400 µm were used for PGSS with a 1 h reaction time. The stability of mackerel oil in the particles did not changed significantly.
Fish oil is derived from the tissues of oily fish, it is recommended as part of healthy diet because it contains ω-3 polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The beneficial health effects of ω-3 PUFAs are well defined: they are essential for the normal growth and development of the brain and nervous system and are also thought to exert beneficial effects during the treatment of coronary artery disease, hypertension, arthritis, clinical depression, anxiety, inflammatory and autoimmune disorders and cancer (Cao and Hur, 2005; Correa et al., 2008; Jeong et al., 2006; Lee et al., 2006; Su et al., 2003; Naliwaiko et al., 2004; Green et al. 2006; Yehuda et al., 2005; Nemets et al., 2002).
Mackerel belongs to the family
Several methods have been reported for extracting fish oils that result in varying yields. Lipids are conventionally extracted and purified using methods such as hexane extraction, vacuum distillation, urea complexation, or conventional crystallization. However, these methods have the disadvantage of requiring high-temperature processing those results in the decomposition or degradation of thermally labile compounds or of employing toxic solvents with adverse health effects (Hultin, 1994; Staby and Mollerup, 1993). Supercritical carbon dioxide (SC-CO2) extraction is a novel and promising process for the extraction and fractionation of edible oils containing labile PUFAs. Using carbon dioxide as the solvent is advantageous, because it is non-flammable, non-toxic, inexpensive, and can be used under mild operating conditions.
The formulation of natural substances together with a biocompatible or biodegradable carrier material to form composites or encapsulates has great potential for the pharmaceutical, cosmetic and food industries (Cocero et al., 2009). Natural substances such as carotenoids, fatty acids and antioxidants are being used extensively in a variety of food products (Budavari, 1989). In addition several clinically approved pharmaceutical products use biodegradable polymers to regulate the rate of drug release within the body (Tracy, 1998; Okada, 1997).
Different processes have been used for encapsulation, including spray-drying, freeze-drying, liquid antisolvent crystallization and milling processes. However, there are several disadvantages to these technologies, such as the production of coarse particles with a broad particle size distribution, product degradation due to mechanical or thermal stress and particle contamination with organic solvents or other toxic substances. Therefore, novel alternative precipitation methods are currently being investigated (Martin and Cocero, 2008).
Particle formations techniques SC-CO2 such as the rapid expansion of supercritical solutions (RESS), particles from gas saturated solutions (PGSS), and supercritical anti- solvent (SAS) precipitation have received much attention as precipitation methods alternative to those using organic solvents (Mishima, 2008). These methods are important for drug delivery systems to successfully obtain composites or encapsulates that comprise an active compound loaded into a matrix of a carrier material, thus improving product preservation as well as controlling the dissolution rate of the active compound (Cocero et al., 2009).
Achieving small particles with a narrow particle size distribution for pharmaceutical agents is a major aim in the design of conventional drug delivery systems such as tablets, capsules, injection. Biphasic drug delivery systems such as suspension and emulsion and controlled drug delivery systems such as implants, transdermal, microemulsions and nanoparticulate are also important for pharmaceutical development (Budavari, 1989; Mishima, 2008; Turk and Lietzow, 2008; Yildiz et al. 2007; Park and Yeo, 2008; Tandya et al. 2006; Li et al., 2006).
PGSS can be used to produce microparticles with a narrow size distribution; therefore, it is a key technique used in the food and pharmaceutical industries, because it results in solvent-free products (Pathak et al., 2006). Therefore, the aim of this study was to extract mackerel oil using SC-CO2 and hexane to compare the fatty acid composition and stability of extracted oil and oil particle.
Mackerels were collected from the Busan Cooperative Fish Market (Seo-gu, Busan, Korea). The muscle was separated mechanically and then washed thoroughly with cold distilled water in the laboratory. Pure carbon dioxide (99.99%) was supplied by KOSEM (Sangbuk-myeon Yangsan, Korea). All other chemicals used in this study were of analytical or HPLC grade.
The mackerel muscle was dried in a freeze-dryer for about 72 h and then crushed using a mechanical blender supplied by DONG YANG PCS CO. LTD. (Ansan, Korea). These “freeze dried mackerel muscle” samples were then stored at -20℃.
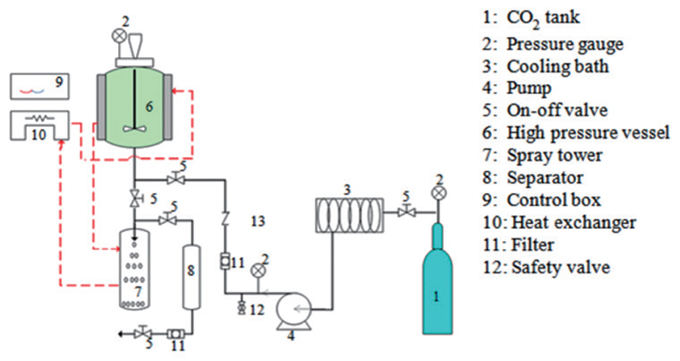
Laboratory scale of supercritical fluid extraction (SFE) was performed. 20 g of freeze dried raw mackerel muscle was loaded into a 200 mL stainless steel extraction vessel. A thin layer of cotton was placed at the bottom of the extraction vessel and a second layer was placed above the sample before plugging the cap. CO2 was pumped into the extraction vessel at a constant pressure using a high pressure pump (Milroyal, Milton Roy, USA) until the desired pressure was obtained. A backpressure regulator was used to control the CO2 pressure. The extraction temperature was maintained by connecting the extraction vessel to a water bath. Flow rates and the accumulated gas volume passing through the apparatus were measured using a gas flow meter (Shinagawa, Tokyo, Japan). After SC-CO2 extraction, the remaining mackerel muscle residues in the vessel and oil was stored at -20℃ until further use and analysis. Mackerel muscle was extracted at a temperature of 45℃ and pressure ranging from 15 to 25 MPa for 2 h using SC-CO2. The flow rates of CO2 were kept constant at 27 g/min for all extraction.
Extraction was performed using hexane as the solvent. 40 g of freeze dried raw mackerel muscle were placed into a beaker with 200 mL hexane and stirred at 300 rpm for 20 h at 45℃. After extraction, the hexane solution was filtered using a filter paper and then evaporated in a rotary vacuum evaporator at 40℃. The oil was then stored at -20℃ until use.
>
Particle formation using PGSS
The experiments were carried out using PEG 8000 (g/mol) and mackerel oil at different pressures and temperatures. A schematic diagram of the PGSS process used in this study is shown in Fig. 1. PGSS experiment began by delivering SC-CO2 to the precipitation chamber until the desired pressure was reached. PEG and mackerel oil (5:1) in the reactor were melted using SC-CO2 and mixed with a stirring wheel at 300 rpm. These experiments were carried out at temperatures of 45 to 55℃ and pressures, ranging from 15 to 25 MPa for 1 h. After the reaction was complete, PEG conjugated materials were delivered through a 400 μm nozzle and collected from a separator.
>
Fatty acid compositions determination
Gas chromatography (GC) analysis was performed to determine the fatty acid composition of mackerel muscle oil obtained by SC-CO2 extraction and after making particle using polyethylene glycol (PEG). The GC–MS analysis was performed using a 6890 Agilent (Agilent Technologies, Wilmington, USA) gas chromatograph with a fused silica capillary column (100 m length × 0.25 mm internal diameter, 0.2 µm of film) (Supelco, Bellefonte, USA). Fatty acid methyl esters were prepared according to official methods and recommended practices of the American Oil Chemists’ Society (AOCS, 1998), using nitrogen as the carrier gas (1.0 mL/min). The oven temperature was programmed to start with a constant temperature of 130℃ for 3 min then increase to 240℃ at a rate of 4℃/min and then hold at 240℃ for 10 min. The temperature of both injector and detector was 250℃. Fatty acid methyl esters were identified by comparing the retention time with a standard fatty acid methyl ester mixture (Supelco, Bellefonte, PA., U.S.A.).
Several parameters determine the deterioration of oil. In this study, the stability of oil before and after particle formation was monitored by assessing the acid value, peroxide levels and free fatty acid content.
The AV was determined according to AOCS official method (AOCS, 1998). 1 g of sample was dissolved in 100 mL ether:ethanol (1:1) with shaking. 4 drops of the indicator phe- nolphthalein were then added. The solution was titrated with 0.1 N KOH-ethanol until it becomes a pink color and the acid value was expressed as mg of KOH per g of sample.
Acid value (AV) = 56.11 * A * F/S
Where A is the volume of KOH-ethanol solution used in the titration (mL), F is the concentration of KOH-ethanol factor, S is the mass of the oil (g) and 56.11 is the molecular weight of KOH.
The peroxide value was determined according to the AOCS official method (AOCS, 1998). 1 g of sample was dissolved in 6 mL acetic acid-chloroform (3:2) solution. Then 0.1 mL saturated potassium iodide solution was added to the mixture, which was then allowed to stand with occasional shaking for 1 min. Distilled water (6 mL) was immediately added to the solution. The solution was titrated with 0.01 N of sodium thiosulfate until the yellow iodine color had almost disappeared. Next 0.4 mL starch indicator solution was added and the solution was titrated again until the blue color disappeared. A blank control was obtained following the same procedure. The peroxide value was expressed as milliequivalents peroxide/1,000 g sample.
Where, S is volume of sample titrant (mL), B is the volume of blank titrant (mL), N is the normality of the sodium thiosulfate solution and M is the mass of sample (g).
>
Free fatty acid (FFA) content
The FFA content of mackerel oil was analyzed according to the method of Bernardez and coworkers (Bernardez, 2005). Briefly, 50 mg oil was placed into Pyrex tubes with 3 mL cyclohexane, and 1 mL cupric acetate-pyridine reagent was added. Tubes were vortexed for 30 s and centrifuged at 2000 × g for 10 min. The absorbance of the upper layer was read at 710 nm. The FFA content of the oil was measured against a calibration curve generated using oleic acid as the standard.
All eperiments were performed in triplicate. Data were analyzed using analysis of variance (ANOVA) and the differences between means were evaluated using Duncan’s multiple range test. The SPSS statistics program (SPSS version 15.0 for Windows, SPSS Inc., Chicago, IL, USA) was used for statistical analysis
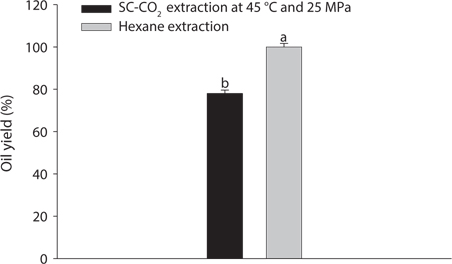
Mackerel muscle oil was extracted using SC-CO2 at a temperature of 45℃ and a pressure ranging from 15 to 25 MPa. The highest oil concentration obtained by SC-CO2 extraction was 4.00 ± 0.11 g/20 g of mackerel muscle at 45℃ with a pressure 25 MPa. When the reactions were performed at a constant temperature, the amount of oil extracted from mackerel muscle increased with increasing pressure. As pressure increased, the density of SC-CO2 also increased, enhancing the solvating power. Bulgarevicg et al., 2002 reported that elevated pressure increased solvent power by strengthening intermolecular physical interactions. Similar results were reported during the extraction of oil from boiled anchovy (Park et al., 2008). The total amount of oil obtained using SC-CO2 extraction in the current study was 20.00 ± 0.54% at 45℃ and 25 MPa, whereas the amount of oil obtained from mackerel muscle using hexane extraction was 25.62 ± 0.62%. Based on the assumption that oil extraction using hexane had completed, the yield of SC-CO2 extracted oil at 45℃ and 25 MPa was 78.06 ± 1.55% shown in Fig. 2. The observed difference in maximum oil yield might be due to variations in the extraction period, processing unit or operating conditions.
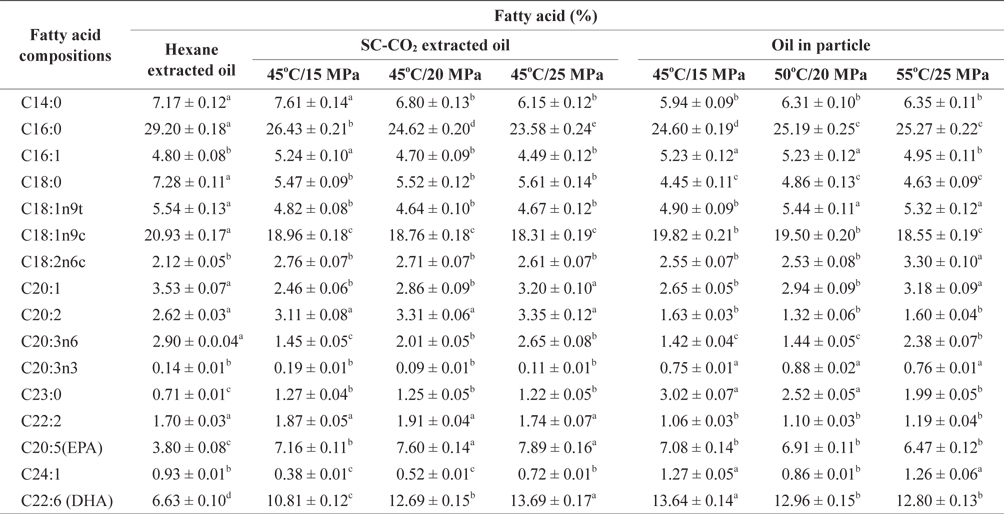
The fatty acid compositions of the oils obtained from SC-CO2 and hexane extraction and PGSS obtained particles are shown in Table 1. Of the saturated fatty acids identified in hexane extracted oil, palmitic acid (C16:0) was present in the highest concentrations, accounting for 29.20 ± 0.18% of the total fatty acids content. The lowest concentration of palmitic acid was found in SC-CO2 extracted oil at 45℃ and 25 MPa, the value was 23.58 ± 0.24% of the total fatty acids. After generating the particles of extracted oil at different temperatures and pressures of SC-CO2, the concentration of palmitic acid not changed significantly. The highest concentration of palmitic acid detected in the particles was 25.27 ± 0.22% at 55℃ and 25 MPa whereas the lowest was 24.60 ± 0.19% at 45℃ and 15 MPa. After increasing the temperature and pressure during particle formation, the concentration of palmitic acid changed only slightly.
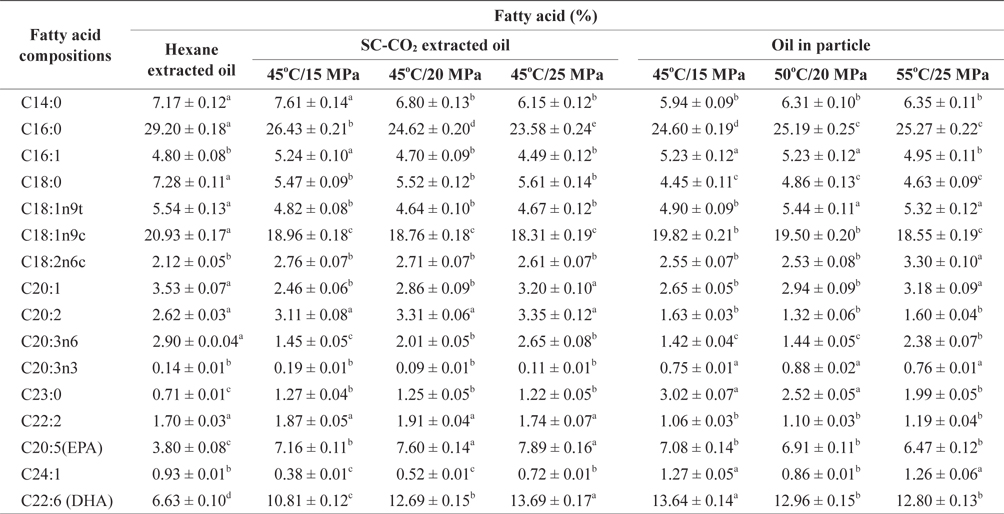
Fatty acid compositions percentage of mackerel muscle oil obtained by SC-CO2 and hexane extraction and oil in particle
Among the monounsaturated fatty acids of extracted oil, oleic acid (C18:1n9c) was also found in substantial concentrations, accounting for 18.31 ± 0.19% to 20.93 ± 0.17% of the total fatty acids identified. There was no significant change in oleic acid concentrations in the oil particles. DHA (C22:6) was present in the extracted mackerel muscle oil at higher levels than were other PUFAs. The highest amounts of EPA (C20:5) and DHA (C22:6) were 7.89 ± 0.16% and 13.69 ± 0.17%, respectively of the total fatty acids in SC-CO2 extracted oil at 45℃ and 25 MPa. In contrast the lowest amounts of EPA (C20:5) and DHA (C22:6) were 3.80 ± 0.08% and 6.63 ± 0.10% respectively, of the total identified fatty acids found in hexane extracted oil.
The composition of total PUFAs in mackerel muscle oil was comparable with those obtained from marine fish oils such as cod liver and anchovy oils which contain about 14-31% of EPA and DHA (AOCS, 1998). Oil extracted using SC-CO2 contained a higher percentage of PUFAs compared with oil extracted using hexane. This might be due to the application of increased temperature and the prolonged extraction period required during hexane extraction compared with SC-CO2 extraction. A Long extraction period the presence of elevated temperature might lead to thermal degradation of fatty acids, particularly unsaturated fatty acids. Similar results were reported when the fatty acid profiles of hake byproducts were assessed (Rubio-Rodriguez et al., 2008).
The percentages of EPA and DHA in particles formed oil were not changed significantly compared with SC-CO2 extracted oil at 45℃ and 25 MPa. This might be due to the short reaction time required for oil particle formation. There was a small change in the amount of EPA and DHA in oil particles formed at different temperatures and pressures. Levels were reduced slightly with increased temperature and pressure, which might be due to temperature sensitivity of EPA and DHA.
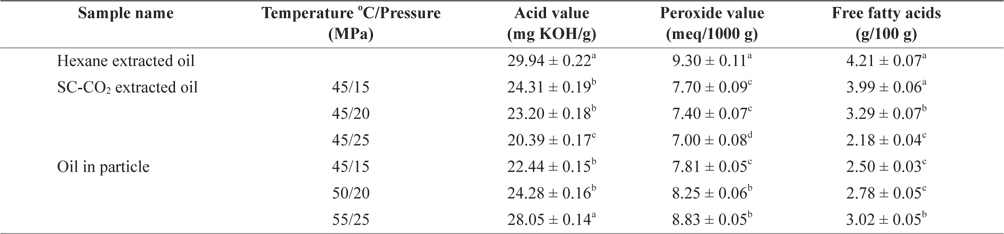
Marine fish oil contains high levels of PUFAs. The quality of oil deteriorates at different rates depending on the production and storage conditions (Kamal-Eldin and Yanishlieva, 2002). The AV, POV and FFA content of oil extracted using SC-CO2, hexane and after particle formation are shown in Table 2. The amount of acid value, peroxide value and free fatty acid content were higher in hexane extracted oil than in SC-CO2 extracted oil. Because the hexane extraction system is open, samples are exposed to increased amounts of oxygen during the extraction explaining the increased oxidation. In contrast, the exposure to low levels of oxygen only during SC-CO2 extraction caused minimal oxidation. The AV was calculated to determine the acidity of oil and a low AV is indicative of a high oxidative stability (Essien et al., 2012). In contrast, the POV of an oil or fat is a measurement of rancidity which is a result of autoxidation. The AV and POV of mackerel muscle oil obtained using different SC-CO2 extraction conditions ranged from 20.39 ± 0.17 to 24.31 ± 0.19 mg KOH/g and 7.00 ± 0.08 to 7.70 ± 0.09 meq/1000 g. AV and POV of soybean oil was found 9.75% and 0.5 meq/g, respectively (Ashaye and Olusoji, 2006) which were higher than those of mackerel oil.
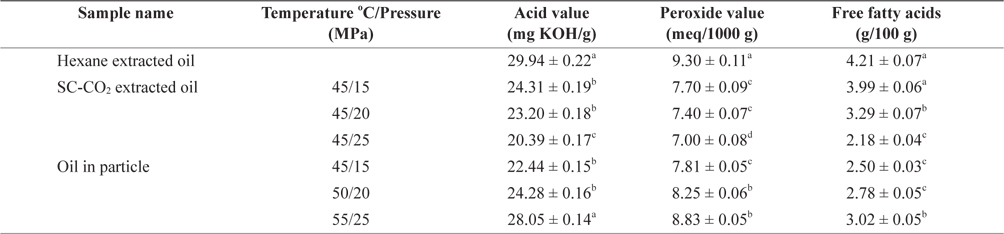
Acid value, peroxide value and free fatty acid content of mackerel muscle oil obtained by SC-CO2 and hexane extraction and oil in particle
FFAs are directly responsible for the acidity of oil. Changes in FFA content are caused mainly by hydrolytic reactions within the oil. The FFA content of mackerel muscle oil extracted using different SC-CO2 conditions ranged from 2.18 ± 0.04 to 3.99 ± 0.06 g/100 g. The AV, POV and FFA contents were reduced using a constant temperature with increasing pressure. However, the oil extracted using SC-CO2 was more stable than that obtained using hexane extraction. It likely that oil extraction using SC-CO2 resulted in less oxidation because oxygen could not penetrate into the closed chamber vessel used for the entire SC-CO2 extraction process. The lowest changes in AV, POV and FFA contents of mackerel oil particles were 22.44 ± 0.15 mg KOH/g, 7.81 ± 0.05 meq/1000 g and 2.50 ± 0.03 g/100 g, respectively, at 45℃ and 15 MPa. With increasing temperature and pressure the AV, POV and FFA contents of mackerel oil particles increased slightly to 28.05 ± 0.14 mg KOH/g, 8.83 ± 0.05 meq/1000 g and 3.02 ± 0.05 g/100 g, respectively at 55℃ and 25 MPa which were not significantly different from the values obtained using SC-CO2 extracted oil at 45℃ and 25 MPa. It likely that mackerel oil particle using PGSS process occurred less oxidation because oxygen could not penetrate into the vessel due to close chamber of whole extraction period.
In conclusion, SC-CO2 extracted oil contained high amounts of PUFAs. The oil quality was not changed significantly after particle formation with PEG, suggesting that it can be used in the food and pharmaceutical industries.