The objective of this study was to evaluate the antiviral activity of Phaeophyta extracts against feline calicivirus (FCV), used as a norovirus surrogate. A bioassay-guided cytotoxicity and virus infectivity assay revealed that methanolic extracts of Phaeophyta possessed significant antiviral activity against FCV. Among them, Eisenia bicyclis extract exhibited the highest antiviral activity against FCV. The 50% effective concentration of the extract (EC50) inhibiting FCV viral replication by 50% was 80 μg/ mL. The extract also showed the highest selectivity index, calculated from the ratio of the median cellular cytotoxicity concentration (CC50) and EC50, indicating antiviral efficacy against FCV. In addition, significant interruption of FCV infection was observed by pretreatment of host Crandall-Reese feline kidney cells with the E. bicyclis extract (200 μg/mL) prior to virus infection, in a dosedependent manner.
In recent decades, norovirus has been reported to cause mass food poisoning (Food Safety and Infection Service, 2009). According to the US Centers for Disease Control and Prevention, ~54 million people develop illness induced by norovirus annually in the United States, and 96% of viral gastroenteritis occurs secondary to the virus (Cheek et al., 2002; Koopmans and Duizer, 2004). In Korea, the first epidemic of food poisoning caused by norovirus occurred in 1999, and norovirus was designated a contagious disease in 2006 (Jee et al., 1999; Ministry of Health and Welfare, 2006). Norovirus can infect the human body through drinking water or food, and is then released into the environment via the vomit or feces. Next, it is transmitted by the ingestion of groundwater and food contaminated with norovirus through the fecal-oral route (Fretz et al., 2005).
Most recent studies of norovirus utilized a norovirus surrogate because norovirus is not cultivatable in laboratory conditions, and appropriate experimental protocols have not been established (Duizer et al., 2004). In this study, a feline calicivirus (FCV) was used as a cultivatable norovirus surrogate (Kim et al., 2009, 2010). Norovirus reportedly shows resistance to several sanitizers (Liu et al., 2010; Park et al., 2010; Centers for Disease Control and Prevention, 2011). Moreover, chemical sanitizers can induce various side effects, such as fever and itching, in humans. Therefore, the development of effective and eco-friendly disinfectants against norovirus is necessary (Malik et al., 2006). Some disinfectant substances originating from natural sources are effective against bacteria and viruses. However, no effective disinfectant against norovirus has been reported. We have focused on Phaeophyta, a potential antimicrobial additive and functional ingredient (Cho et al., 1990; Smit, 2004; Kim et al., 2010). In this study, we reported the antiviral activity of Phaeophyta extracts against norovirus using FCV as a surrogate.
Phaeophyta samples were obtained from a commercial market located in Busan, Korea- in September 2012. Each sample was dried at 40℃ for 2 days and then finely powdered. Each powder (100 g) was exhaustively extracted with aqueous 90% methanol (v/v) at 70℃ for 3 h. The methanolic extract was filtered and concentrated by rotary evaporation at 40℃. The concentrated extract was dissolved in dimethyl sulfoxide to a concentration of 10 mg/mL.
Crandall-Reese feline kidney cells (CRFK cells, ATCC CCL-94) and FCV (ATCC VR-782) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). CRFK cells were grown in Dulbecco’s modified Eagle’s medium (Gibco/BRL, Green Island, NY, USA) as described by Kim et al. (2010). FCV was propagated in CRFK cells and harvested by freeze-thawing. The virus suspension was stored at -80℃ until use.
The cytotoxicity of Phaeophyta extracts was determined by quantifying the viability of CRFK cells using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) assay as described by Kim et al. (2010). The median cellular cytotoxicity concentration (CC50) that resulted in the death of 50% of CRFK cells was determined by MTT assay.
The antiviral activity of Phaeophyta extracts against FCV was evaluated in terms of the tissue culture infectivity dose 50 (TCID50), as described by Kim et al. (2010). The antiviral effective concentration was expressed as the EC50 value, defined as the concentration of the sample required to inhibit virus-induced cytopathogenic effects (CPEs) by 50% (Bidawid et al., 2003). In brief, the FCV suspension (log 5 TCID50 per mL) was treated with identical volumes of serially diluted extracts at room temperature for 24 h. The mixtures were added to a monolayer of CRFK cells in a 96-well plate. After incubating at 37℃ for 2 to 3 days, the cells were stained with crystal violet solution and monitored for CPEs. Untreated controls were suspended with maintenance medium instead of extract. The selectivity index (SI) was calculated as the ratio of CC50 to EC50 for each compound. The SI indicates the efficacy of antiviral activity. A higher SI means greater antiviral activity with low cellular toxicity, as well as higher commercialization potential (Oh et al., 2013).
>
Pretreatment effect of Phaeophyta extracts prior to FCV infection
CRFK cells were treated with Phaeophyta extracts and incubated for 24 h at 37℃ prior to virus infection. The effect of Phaeophyta extract on FCV infection was then evaluated in terms of the TCID50 values, as described above.
>
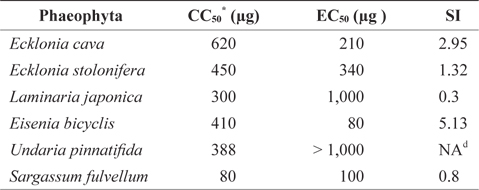
Antiviral activity of Phaeophyta extracts against FCV
Our previous study suggested that some seaweeds exhibit antiviral activity against FCV (Kim et al., 2010). In this study, we evaluated the antiviral activity of Phaeophyta methanolic extracts (Table 1). The cytotoxicity of the methanolic extracts against host CRFK cells was first determined by MTT assay and reported as the CC50 value. As shown in Table 1,
[Table 1.] Antiviral activity of Phaeophyta methanolic extracts against feline calicivirus
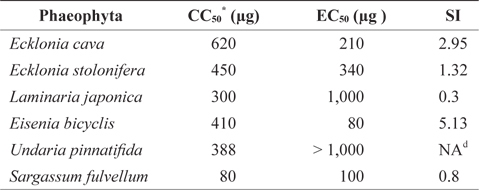
Antiviral activity of Phaeophyta methanolic extracts against feline calicivirus
The antiviral activities of Phaeophyta extracts against FCV were measured by TCID50 assay, and expressed as EC50 values. The
>
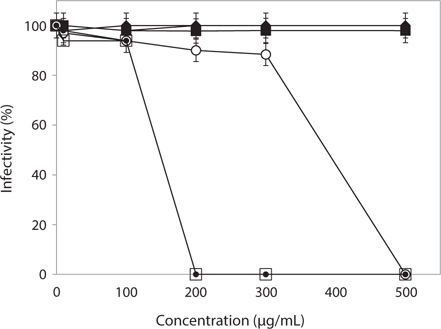
Protective effect of Phaeophyta extracts on FCV infection
To evaluate the protective effect of Phaeophyta extracts on FCV infection, CRFK cells were treated with various concentrations of methanolic extracts prior to virus infection. As shown in Fig. 1, FCV infectivity was completely interrupted by treatment with 200 μg/mL
Several reports of protection against viral infection have been published. Cho et al. (2013) reported a protective effect of red ginseng extract against vaginal herpes simplex virus infection, which was mediated by promotion of host defence systems. In addition, Carlucci et al. (2004) reported a protective effect of carrageenans against genital herpes simplex virus infection, which was mediated by interruption of viral replication. Although the precise mechanism of the protective effect against FCV infection has not yet been defined, our results suggested that
To our knowledge, this is the first report of the protective effect of Phaeophyta extract against infection with a norovirus surrogate. Our results will contribute to the development of a food contact sanitizer effective against norovirus because Phaeophyta extracts can be used as a food additive.