Glufosinate-ammonium (Basta; Bayer CropScience, Monheim am Rhein, Germany), a nonselective (broad-spectrum) herbicide, is extensively used to control a wide range of weeds in agricultural and industrial areas (Cox, 1996; Orme and Kegley, 2006). It is used as a preharvest desiccant for a variety of crops, including potatoes, peas, beans, and cereals (PAN UK, 1998), and it also being increasingly used as a selective herbicide on transgenic crops that have been genetically modified to tolerate it (Bayer CropScience, 2007; Duke and Cerdeira, 2010). Glufosinate-ammonium acts by blocking the activity of an enzyme involved in the synthesis of the amino acid glutamine (Hoerlein, 1994; Cox, 1996), and its inhibition of glutamate synthetase has been reported not only in plants but also in animals and humans (Cox, 1996; HSD, 2003). The World Health Organization (WHO) classifies glufosinate as an acute hazard (class III), and the United States Environmental Protection Agency (U.S. EPA) classifies it as “persistent” and “mobile.” However, it is registered for use in over 80 countries (Bayer CropScience, 2005) and is sold under several trade names, including Basta, Rely, Finale, Ignite, Challenge, and Liberty (Jewell and Buffin, 2001).
The exposure of nontarget aquatic organisms to glufosinate formulations by pollution of rivers and marine environments through runoff is a concern because the compound is highly water-soluble and extensively used (Cox, 1996; Jewell and Buffin, 2001). Glufosinate has been detected in soils (EFSA, 2005), groundwater (HSD, 2003; EFSA, 2005), and surface waters and sediments (KEMI, 2005). Tests of the effects of an herbicide containing glufosinate-ammonium on aquatic organisms (sheepshead minnow
Histopathological biomarkers can be used in fish species to assess the biological effects of contaminants such as agrochemicals (Van der Oost et al., 2003). The utility of histological lesions as sensitive and reliable indicators of fish health has been reported in previous studies (Stentiford et al., 2003; Bernet et al., 2004; Ramírez-Duarte et al., 2008). Numerous studies of glyphosate-based herbicides (e.g., Roundup) have demonstrated their toxic effects on fish (the Nile tilapia
The marine medaka
As a first step in examining the toxic effects of glufosinate-containing herbicides (Basta) in aquatic environments at various salinity levels, we evaluated histological lesions in the liver and gills of marine medaka in freshwater in response to subchronic and chronic exposure to Basta for 28 and 42 days, respectively.
The marine medaka specimens used in this study were from a laboratory stock maintained at the Institute of Marine Living Modified Organisms, Pukyong National University, Busan, Korea. Their mean total length was 10.1 ± 0.6 mm and their mean weight was 10.2 ± 2.0 mg.
>
Short-term toxicity (LC50) testing
short-term static toxicity test was performed to estimate the median lethal concentration of Basta on marine medaka exposed for 96 h (96 h-LC50). The Basta used in the present study contained 18.02% glufosinate-ammonium as the active ingredient and 9.91% surfactants (Bayer CropScience, 2013). The fish were exposed to the following nominal concentrations of Basta: 0.0, 7.5, 8.0, 8.5, 9.0, 9.5, or 10.0 mg/L. The control and each experimental concentration were tested in duplicate with 12 individuals per group in 2-L aquariums. The individuals were starved for 24 h before the experiment and were not fed during the experiment. Fish showing no respiratory movement and no response to tactile stimuli were considered dead and were removed immediately. The 96 h-LC50 was computed using probit and logit analyses.
>
Subchronic and chronic toxicity testing
For the subchronic and chronic toxicity tests, the fish were exposed to different concentrations of Basta (2, 4, or 8 mg/L) for 28 and 42 days, respectively. The experimental groups and control groups, each containing 65 fishes, were assigned to an aquarium and the tests were performed in duplicate. The fish were maintained in 10-L aerated glass aquariums containing dechlorinated tap water at a temperature of 25 ± 1℃, with a 12 h:12 h photoperiod. They were fed twice daily with a commercial diet (Ewha Oil Co., Busan, Korea), and one-fourth of the water in each aquarium was replaced daily throughout the experiments. All the procedures in this study were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NRC, 2011). The number of dead fish was recorded and they were promptly removed. After exposure, the fish were anesthetized with ice-cold water and fixed in 10% formaldehyde for over 24 h. The fish were dehydrated through a graded series of ethanol and cleared in xylene. They were embedded in paraffin, sectioned to a thickness of 5 μm, and stained with hematoxylin and eosin. Tissue lesions were examined under a light microscope (E400; Nikon Co., Tokyo, Japan) and photographed with a digital camera (Moticam Pro 205A; Motic Co., Hong Kong, China).
For the histological analysis of the gills, five filaments were examined per individual fish. To calculate the percentage of filaments affected, the number of secondary lamellae displaying a particular change was recorded and divided by the total number of gill secondary lamellae examined. To evaluate histological changes in the liver, the middlemost section of the liver was used because the type of lesion was the same throughout all livers. The type of change and its extent were recorded for each area and divided by the total number of areas examined, and the values for the affected liver areas were converted into percentages.
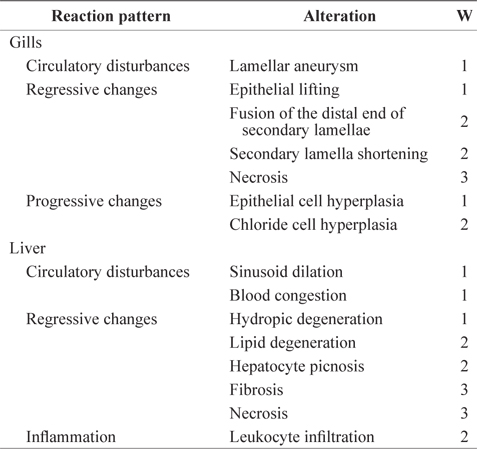
The histopathological lesions were assessed according to the standardized assessment tool used in the protocol described by Bernetet al. (1999) and Hued et al. (2012). Each histological alteration was classified into one of four reaction patterns: circulatory disturbances, regressive changes, progressive changes, or inflammation. Circulatory disturbances (reaction pattern 1 = Rp1) corresponded to pathological conditions of the blood and tissue fluid flow; regressive changes (reaction pattern 2 = Rp2) corresponded to processes that caused a functional reduction or loss of an organ; progressive changes (reaction pattern 3 = Rp3) corresponded to processes that led to increased cell or tissue activity; and inflammation (reaction pattern 4 = Rp4) corresponded to inflammatory processes associated with the other reaction patterns, such as the infiltration of leukocytes (Table 1). An importance factor (W; range, 1 to 3) was assigned to each alteration because the relevance of a lesion depends on its pathological importance. A high importance factor indicated that the change had a greater potential to affect fish health (Bernet et al., 1999). For both the gill and liver tissues, the percentages of gill filaments or liver areas affected were evaluated using a score ranging from 0 to 6 based on the degree and extent of a particular alteration: 0 = unchanged, 2 = mild occurrence, 4 = moderate occurrence, and 6 = severe occurrence. The indices for each alteration were summed to give an index for each reaction pattern (RPOrg = reaction pattern index for an organ). The indices for each reaction pattern of an organ were summed to give an overall organ index (HIGills = gill histopathological index; HILiv = liver histopathological index). The total histopathological index (HITot) was calculated by adding the gill and liver indices of an individual fish (Bernet et al., 1999): the greater the index value, the more severely the organs were affected.
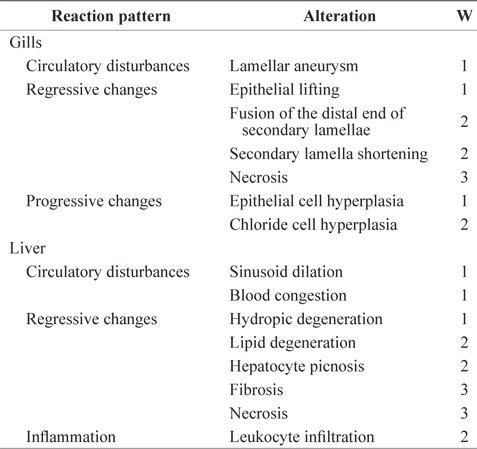
Importance factors for histological gill and liver alterations for each reaction pattern used in this experiment
The differences in the biological parameters at different Basta concentrations and for times of exposure were assessed with Student’s
>
Toxicity test for the 96 h-LC50
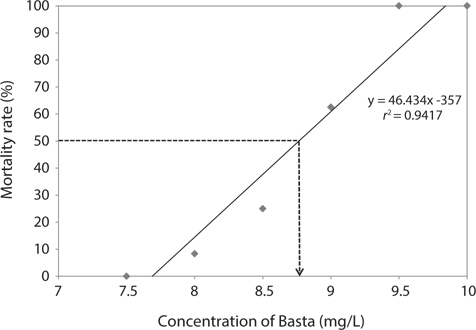
We estimated the 96 h-LC50 value for Basta on the marine medaka as 8.76 mg/L (Fig. 1). The linear relationship between the probit mortality and the concentration of Basta indicated a positive correlation. The mortality rate of the exposed fish increased as the concentration of glufosinate increased. No mortality occurred in the control group or in the groups exposed to 7.0 and 7.5 mg/L Basta, whereas all individuals exposed to 9.5 and 10 mg/L died during the bioassay period.
>
Subchronic and chronic toxicity test
The mortality rate of fish exposed to Basta increased as the exposure period increased, whereas it did not correlate positively with the increased concentrations of Basta (2 mg/L: 28 days, 39.23 ± 5.44%; 42 days, 69.09 ± 7.71%; 4 mg/L: 28 days, 37.69 ± 1.09%; 42 days, 59.09 ± 3.86%; 8 mg/L: 28 days, 56.15 ± 3.26%; 42 days, 71.54 ± 5.44%). No mortality was recorded in the control fish for the duration of the experiment.
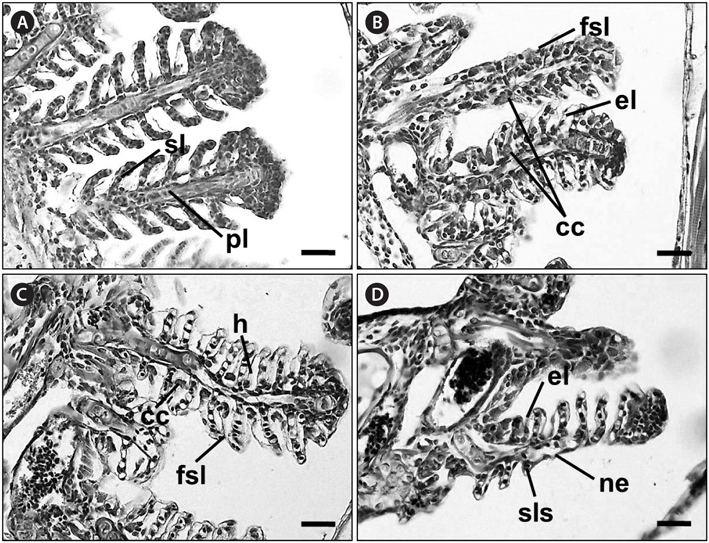
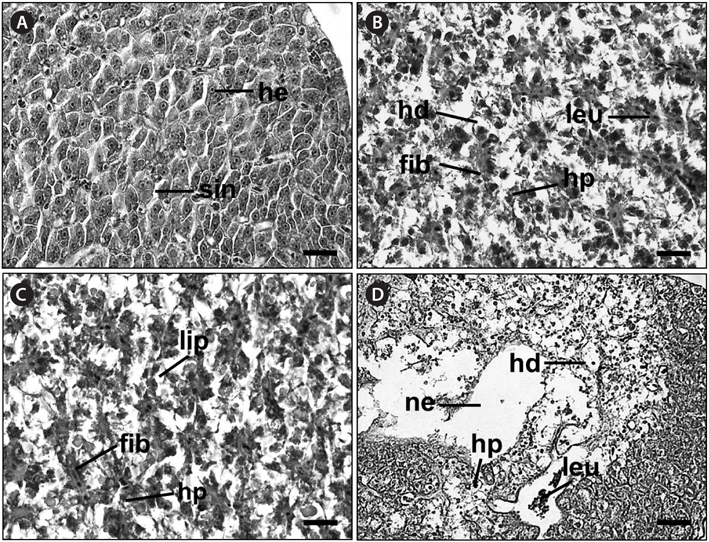
No recognizable changes were observed in the gills of the control group. The primary lamellar epithelium was 1-2 cell layers thick. The secondary lamellae consisted of one layer of epithelial cells (Fig. 2A). However, the treated groups exposed to glufosinate-ammonium showed several pathological changes, the severity and frequency of which increased as the concentration of Basta increased. Individuals exposed to 2 mg/L Basta for 28 days displayed lifting of the secondary lamellar epithelium, fusion of the distal ends of the secondary lamellae, and chloride cell hyperplasia (Fig. 2B). Individuals treated with 4 mg/L Basta for 28 days showed severer secondary epithelial lamellar than fish exposed to 2 mg/L. Compared with the control group, they also presented folding and fusion of the secondary lamellae, and hypertrophy of the epithelial cells (Fig. 2C). Fish exposed to 8 mg/L Basta for 42 days showed severe necrosis and shortening of the secondary lamellae, slight thickening of the primary lamellae, and several areas of edema. Aneurysms of the secondary lamellae, presenting as club-shaped distal portions of the secondary lamellae, were also observed and the primary lamellae were almost indistinguishable from the secondary lamellae (Fig. 2D).
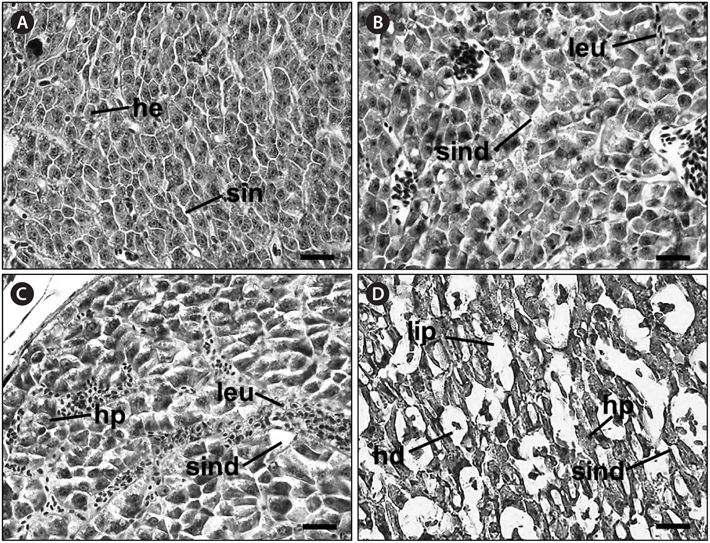
Histological analysis of the livers of the control fish showed a normal parenchymatous appearance (Figs. 3A and 4A). In the control fish livers, hepatocytes were homogeneous in both size and cytoplasm density. However, the livers of the exposed fish displayed various concentration-dependent changes after both 28 and 42 days. The livers from individuals exposed to 2 mg/L Basta for 28 days showed blood sinusoid dilation and some leukocyte infiltration (Fig. 3B), whereas those treated with 4 mg/L Basta showed hepatocyte pyknosis, sinusoidal dilation, and leukocyte infiltration (Fig. 3C). In individuals treated with 8 mg/L Basta, not only were all the alterations observed in the 2 and 4 mg/L-treated groups more severe and more frequent, severe lipid degeneration, hydropic degeneration, and necrosis were also present (Fig. 3D).
In the livers of fish exposed to Basta for 42 days, individuals treated with 2 mg/L Basta showed hydropic degeneration and hepatocyte pyknosis, together with severe leukocyte infiltration and fibrosis (Fig. 4B). The livers of fish exposed to 4 mg/L Basta presented all the changes mentioned above and lipid degeneration (Fig. 4C). In individuals treated with 8 mg/L Basta, hydropic degeneration, hepatocyte pyknosis, and leukocyte infiltration were severe, and necrosis was observed only in this group (Fig. 4D).
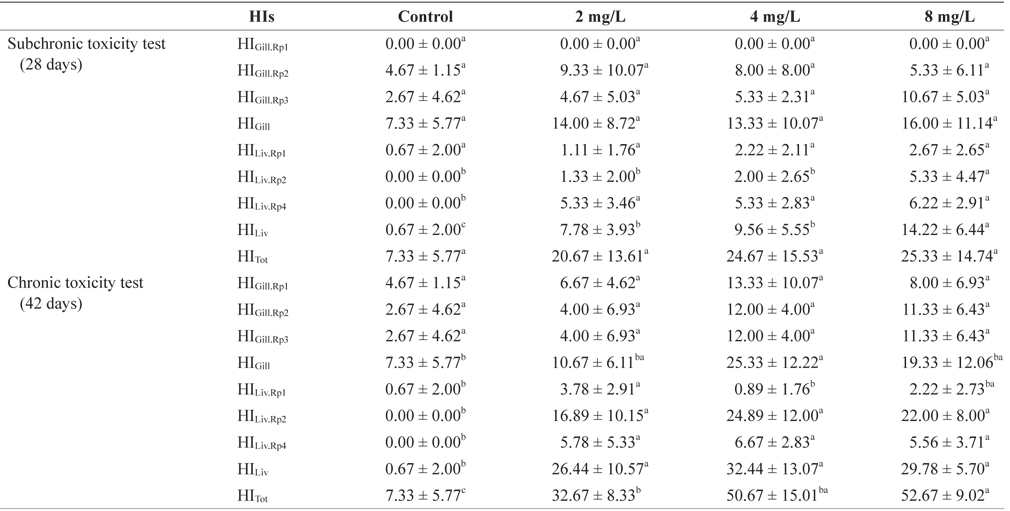
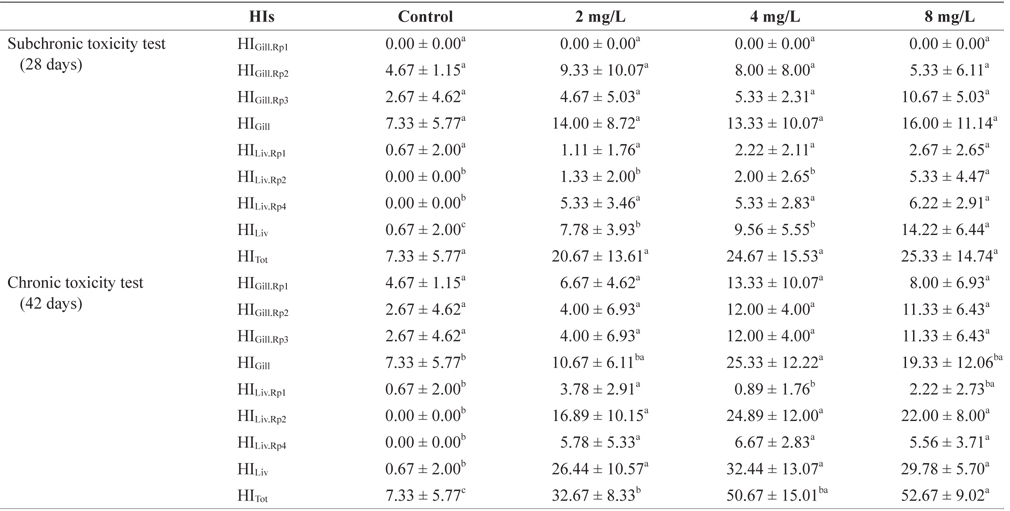
A summary of the histopathological indices (HIs) for the gills and livers of the marine medaka subjected to different concentrations of Basta for 28 and 42 days is presented in Table 2.
Histopathological indices (HIs) after subchronic and chronic exposure to Basta on the marine medaka Oryzias dancena
The gill HIs for individual fish exposed to Basta varied according to the concentration tested (Table 2). The HIGill·Rp1 (circulatory disturbances), HIGill·Rp2 (regressive changes), and HIGill·Rp3 (progressive changes) values did not differ significantly among the treatment and control groups at either 28 or 42 days (
The liver HIs of individual fish exposed to Basta varied according to the concentration tested (Table 2). The liver histological analyses showed HILiv·Rp1 values that indicated relevant circulatory changes, and these not only differed significantly between the treatment groups and the control group, but also among the treatment groups. The HILiv·Rp2 values (regressive changes) differed between the control group and the 8 mg/L-treated group, whereas those of the other groups did not differ from the control value at 28 days. However, after 42 days of exposure, the HILiv·Rp2 values of the treatment groups were significantly different from those of the control group but did not vary among the treatment groups (
The total gill HI did not differ among the treatment groups or between the treatment and control groups at 28 or 42 days. The total liver HI differed significantly between the treatment and control groups, and also between the 8 mg/L-treated group and the other treatment groups after exposure for 28 days (
In this study, we investigated the toxicological effects of the agrochemical component glufosinate using histopathological changes induced in the marine medaka. In previous studies, glufosinate-ammonium was reported to cause the death of nerve cells in the brains of mammals, both
No literature exists on the histopathological effects of Basta, although numerous studies of the effects of other herbicides, such as glyphosate (Roundup), have been performed on fish with histopathological analyses. In a recent histopathological study, Hued et al. (2012) tested the acute and subchronic toxicity of Roundup by evaluating the histological gill and liver lesions in
Changes in the gill epithelium are considered a good indicator of the effects of many compounds because gills have a large surface area and are located externally (Ballesteros et al., 2007; Ayoola, 2008; Albinati et al., 2009). In marine medaka exposed to glufosinate-ammonium in this study, the severity of the gill alterations in the fish was dependent on concentration and duration. The major changes in the gills were the lifting of the secondary lamellar epithelium, fusion of the distal ends of the secondary lamellae, and chloride cell hyperplasia. Hyperplasia was one of the most frequent types of damage, causing thickened epithelial layers and the fusion of adjacent secondary lamellae. These changes reduce the respiratory surface and increase the toxicant–blood diffusion distance (Cengiz and Ünlü, 2003), which can cause asphyxiation in the fish.
The HIGill values based on the reaction patterns did not increase in individuals exposed to greater concentrations of glufosinate-ammonium. The HIGill·Rp1 values did not differ significantly between the control and treatment groups, or among the treatment groups, whereas the HIGill·Rp2 values (regressive changes) increased, mainly attributable to the high frequency of epithelial lifting and shortening of the secondary lamellae. The HIGill·Rp3 values increased in the groups exposed to 4 and 8 mg/L Basta because the epithelial hypertrophy, hyperplasia, and lifting were severe. These changes were caused by cell activity in an attempt to protect the internal tissues exposed to the toxicant and could lead to reduced gill function (Hintonet al., 2001 ).
Because the liver plays a central role in the circulatory system, it is considered the target organ of chemically induced tissue damage (Hinton et al., 2001). In previous studies, the exposure of fish to an atrazine herbicide increased the size of lipid droplets and vacuolization in the liver (Biagianti-Risbourg and Bastide, 1995), and hydropic degeneration, blood sinusoid dilation, leukocyte infiltration, and lipidic vacuolization have also been reported in fish exposed to commercial formulations of glyphosate (Jiraungkoorskul et al., 2002, 2003; Albinati et al., 2007, 2009; Ayoola, 2008; Langiano Vdo and Martinez, 2008; Hued et al., 2012). The livers of marine medaka exposed to different Basta concentrations showed larger hepatocytes with pyknotic nuclei, leukocyte infiltration, and hydropic degeneration. The HILiv values increased in individuals exposed to higher concentrations of Basta. The HILiv·Rp1 values (circulatory disturbances) increased with increasing blood sinusoid dilation and congestion. The HILiv·Rp2 values (regressive changes) increased with increases in the extent and frequency of hydropic and lipid degeneration, fibrosis, and necrosis, and the HILiv·Rp4 values (inflammation) increased with the increase in leukocyte infiltration. The total values of HILiv for fish treated with 8 mg/L Basta were lower than those for fish treated with 4 mg/L Basta in the chronic toxicity test. This is probably attributable to leukocyte infiltration (HILiv·Rp4), which was mainly observed in the groups exposed to 8 mg/L Basta, although the 4 mg/L exposure group showed not only leukocyte infiltration but also all the liver histological changes included in the HILiv·Rp1 and HILiv·Rp2 patterns. Blood congestion, some areas of leukocyte infiltration, and sinusoid dilation were present in the groups exposed to low Basta concentrations. Leukocyte infiltration is a normal process that removes dead cells from necrotic areas (Crawford, 2000), and enhanced blood flow to the liver, which is necessary for the biodetoxification activity of hepatocytes, leads to blood congestion and sinusoid dilation (Neškovic et al., 1996). Necrosis probably resulted from the excessive work required by the process of detoxification in the liver. These alterations became more severe and more frequent as the concentration of Basta and the period of exposure increased. The histological changes discussed above are frequently associated with agrochemical toxicity (Ballesteros et al., 2007; Albinati et al., 2009). Histopathological analysis of the gill and liver tissues of the exposed marine medaka showed that the liver was the more affected organ when the toxicity of Basta was evaluated.
This research shows that exposure to glufosinate-based herbicides for 28 or 42 days causes a diversity of histological changes in the gills and liver of marine medaka. The use of HIs allowed us to quantify the histopathological lesions in fish exposed to different concentrations of the herbicide. Therefore, the responses recorded here may be useful as diagnostic indicators of the toxicity of glufosinate-ammonium in this species. Further research is required to examine the toxic effects of glufosinate-ammonium-based herbicides in aquatic environments at various salinity levels.