Eyestalk ablation (ESA) is commonly used in aquaculture to stimulate ovarian maturation in crustaceans, and methyl farnesoate (MF) affects crustacean molting and reproduction. To investigate the physiological effects of ESA and MF treatments on the shrimp Litopenaeus vannamei, we compared the effects of single eyestalk removal and MF injections. The ESA group had the lowest survival rate (50%), and individuals in the 0.1 μg and 1.0 μg MF-treated groups had survival rates of 80 and 73.3%, respectively. Conversely, molting numbers were highest in the ESA group, and similar to those of the 1.0-μg MF group. To investigate shrimp growth, we measured body weight during the experimental period and found that individuals in the ESA and 1.0 μg MF groups showed significant increases in body weight. Furthermore, to investigate the effects of ESA and MF treatments on gonadal maturation, the gonad somatic index (GSI) was calculated after the experiment. All treated groups (ESA and MF) had higher GSI values than the control group, but the ESA and 1.0 μg MF groups were not significantly different. Using histological ovary analysis, we determined that all treated groups showed indications of the previtellogenic stage, unlike the control group (immature stage). These results suggest that the high-MF-concentration treatment produced effects similar to those of ESA with respect to molting number, growth, and ovarian maturation.
Manipulating reproduction is a major challenge in crustacean aquaculture. Single-eyestalk ablation (ESA) is currently the only strategy used to induce gonad development and spawning in crustacean aquaculture farms (Choy, 1987; Emmerson, 1980), and is based on the hormonal physiology of decapod crustaceans. Molting and reproduction are regulated mainly by ecdysteroids, especially 20-hydroxyecdysone (20E), which is produced by the Y-organ (Nakatsuji et al., 2009). The eyestalk ganglia contain the X-organ/sinus gland (XO-SG) complex, which produces a molt-inhibiting hormone (MIH). As a member of the crustacean hyperglycemic hormone (CHH) subfamily II, the MIH peptide hormone acts on the Y-organ, suppressing 20E synthesis (Covi et al., 2009; Nakatsuji et al., 2009; Mykles, 2011; Nagaraju, 2011). As the result of ESA, inhibitory factors, including MIH, are removed, increasing 20E production and up-regulating its physiological response. Although ESA is an effective strategy for inducing maturation, the high death rates following surgical procedures, and lower healthy seed ratios, remain major problems (Emmerson, 1980; Choy, 1987); thus, a better strategy must be developed.
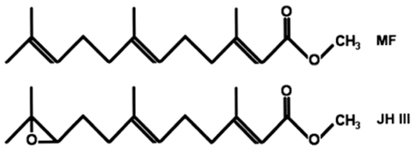
Methyl farnesoate (MF) is a sesquiterpenoid compound found in decapod crustaceans, and is structurally similar to the juvenile hormone (JH) of insects. However, MF differs from juvenile hormone (JH III) in containing an epoxide moiety at the terminal end (Fig. 1). Crustaceans appear to lack epoxidase and S-adenosyl-methionine-dependent methyltransferase, which convert farnesoic acid (FA) to JH III (Hui et al., 2010). Therefore, crustaceans lack JH III, and MF is the end product of sesquiterpenoid biosynthesis. In insects, JH III is the major hormone related to metamorphosis, gonad maturation, and molting (Belles et al., 2005; Tsubota et al., 2010). Since its discovery in the mandibular organ (MO) in crustaceans ~25 years ago, research has accumulated and authors have proposed that MF acts as a crustacean hormone (Borst et al., 1987; Laufer et al., 1987; Nagaraju, 2007). The major production site for MF is MO, and its biosynthesis is negatively controlled by the mandibular organ-inhibiting hormone (MOIH), secreted from the XO-SG complex at the terminal end of the eyestalk (Borst et al., 2001; Nagaraju et al., 2003, 2005). The MOIH peptide hormone suppresses the production of MF.
Previous studies have suggested that MF treatment results in a physiological response similar to that to 20E in decapod crustaceans. Several studies showed that MF stimulates gonadal development in crustaceans (Wainwright et al., 1996; Homola and Chang, 1997; Borst et al., 2002; Nagaraju, 2007). In addition, MF treatment causes molt acceleration in several crustacean species, such as the crayfish
The main goal of this study was to compare the chronic effects of MF treatment and ESA on the growth and ovarian maturation of the shrimp
Live
>
MF treatment and biological measurement
We produced a stock solution of MF (Echelon Biosciences, Salt Lake City, UT, USA), following Nagaraju and Borst(2008) . The solution was aliquoted and stored at –20℃ before use. Sixty immature adult shrimp (body weight [BW] 21.22 ± 1.48 g, mean ± SD) were divided randomly into four groups (15 individuals per group): control, ESA, 0.1-μg MF, and 1.0-μg MF. Eyestalks were removed and wounds were cauterized to minimize hemolymph loss, as described by Salma et al. (2012). The MF solutions were injected into shrimp on days 1, 8, 15, 22, and 29. To calculate survival rate, we removed dead individuals from each experimental group, and to calculate molt frequency, we removed molted shells from tanks, on a daily basis.
After 31 days of culture, individuals in each group were dissected immediately following BW measurement. Survival rate was calculated as the final number of live shrimp × 100/the initial number of shrimp. The BW gain was calculated by comparing the final and initial BWs of individuals. To investigate maturation, we calculated the GSI, as follows. The reproductive organs (ovaries) in the cephalothorax were dissected, following Bell and Lightner (1988), and wet weights were recorded. The GSI was calculated using the standard formula:
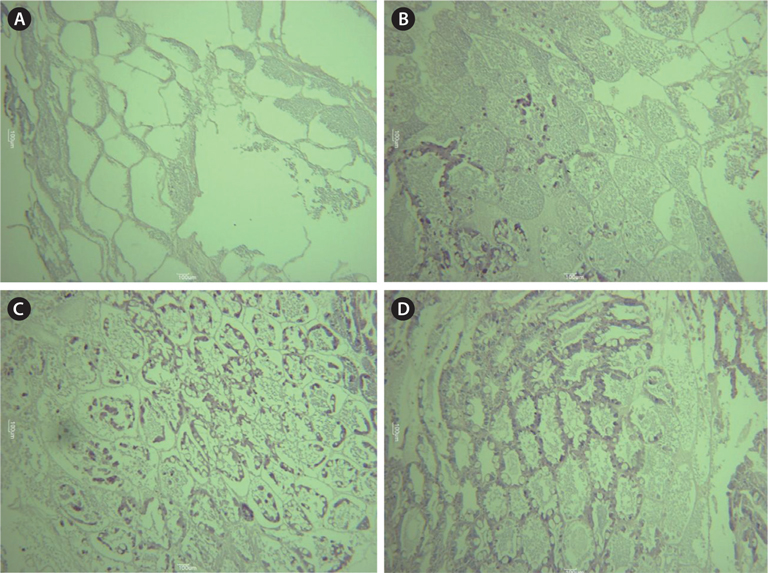
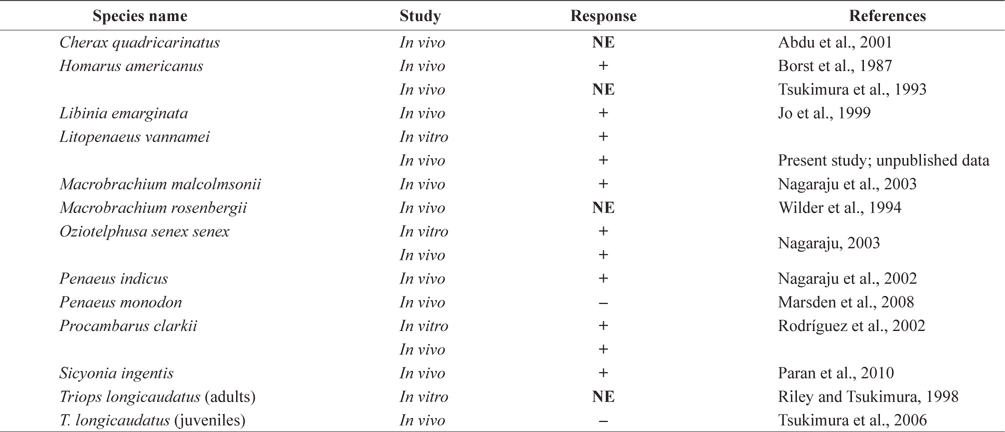
To understand maturation stages, three gonads from each group were subjected to histological analysis. The dissected gonads were fixed in Bouin’s fixative for 1 day and washed in running tap water. After washing, the samples were dehydrated in a gradient alcohol series, and embedded in paraffin. The paraffin blocks were trimmed and then sectioned (5 μm) with a microtome. Sections were stained with Harris’ hematoxylin and eosin, and mounted on a slide using marynol (Muto Pure Chemicals Co., Tokyo, Japan). The developmental stage was divided into the following four categories, following Tinikul et al. (2011): 1) early previtellogenic oocytes, 2) late previtellogenic oocytes, 3) early vitellogenic oocytes, and 4) mature oocytes.
For statistical analysis, we used Student’s
>
Effects of treatments on survival rate, molting and growth
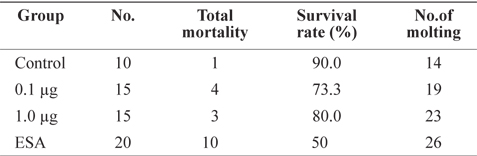
To investigate the effects of ESA and MF treatments, survival rate and molt frequency were calculated after 31 days of culture (Table 1). Survival rate was highest in the control group (90%), followed by MF-treated groups (73.3% for 1.0 μg and 80% for 0.1 μg). The highest mortality occurred in the ESA group (50%), in which six individuals died within 24 h, supporting the notion that physical amputation is a direct cause of death. Although ESA is commonly used for ovarian maturation (Browdy, 1992), it has negative effects on survival. Unilateral ESA is an accepted alternative method of improving survival rates (Ponnuchamy et al., 1981), but rates vary among species; e.g.,
[Table 1.] Effects of MF and unilateral ESA on survival and molting of Litopenaeus vannamei
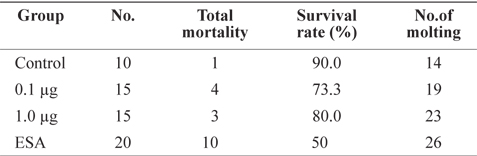
Effects of MF and unilateral ESA on survival and molting of Litopenaeus vannamei
Individuals in the ESA group showed the highest rate of molting, almost double that of the control group (Table 1). Groups treated with MF had a higher frequency of molting than did the control group, and shrimp treated with 1.0 μg of MF showed a frequency of molting similar to that of the ESA group, indicating that MF injection facilitates molting, similar to the effects of ESA. Several previous studies have proposed that MF is associated with crustacean molting. For example, in
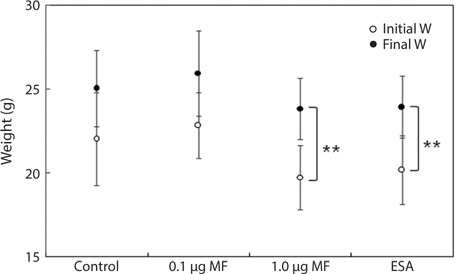
Finally, we evaluated BW gain during the experimental period (Fig. 2). Final BWs in the 1.0-μg MF and ESA groups were significantly different from the initial values (
>
Effect of treatments on ovarian maturation
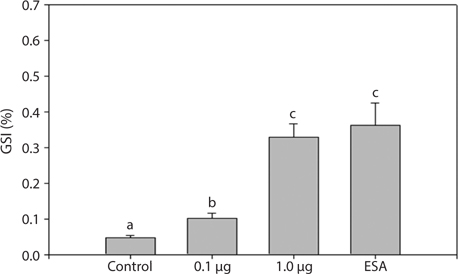
To understand the effects of MF on maturation, GSI indices were calculated 31 days after treatment (Fig. 3). All experimental groups had higher GSI values than the control group. The 1.0 μg MF group (0.33 ± 0.08) had GSI values threefold greater than the 0.1 μg MF group (0.10 ± 0.03), and was not significantly different from the ESA group (0.36 ± 0.14). Therefore, a high level of MF can induce ovarian maturation to the levels induced by ESA.
Histological analysis also indicated that both MF injection and ESA facilitated ovarian maturation (Fig. 4). Unlike in the control group (immature stage), characteristics representative of the previtellogenic stage, with oocytes containing small yolk granules, were found in both the 1.0 μg MF and ESA groups (Tinikul et al., 2011). In the 0.1- and 1.0-μg MF groups, early previtellogenic characteristics, in the form of small follicle cells, were also present and distributed around the periphery of the lobes (Fig. 4). The effects of MF vary among species. No stimulatory effects have been found in
[Table 2.] Effects of methyl farnesoate on crustacean reproduction
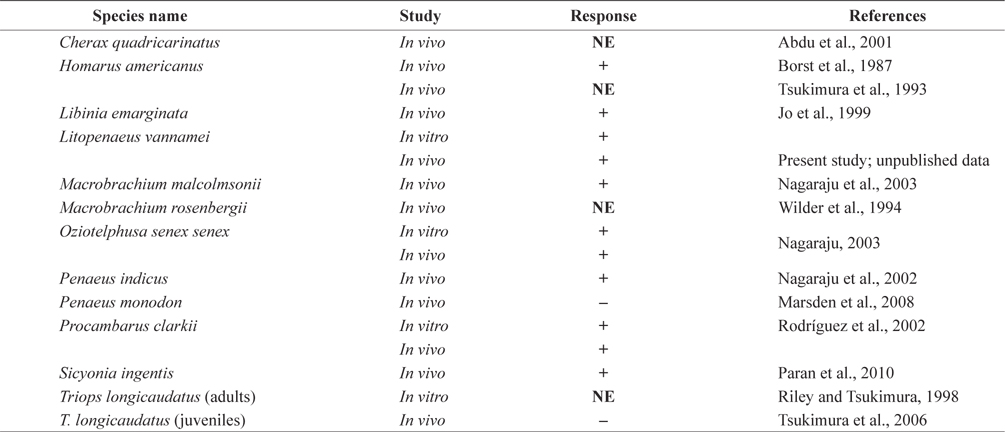
Effects of methyl farnesoate on crustacean reproduction
The regulation of growth and ovarian development processes in crustaceans are related to the molting cycle. Molting regulation in crustaceans is known to be controlled primarily by ecdysteroids (Wongsawang et al., 2005; Brown et al., 2009). The Y-organ is responsible for the synthesis and secretion of the inactive precursor compound, ecdysone, which is converted to 20E, the biologically active ecdysteroid (Chang, 1985). This process is negatively regulated by the MIH, which is also secreted from the XO/SG complex (Webster, 1998; Huberman, 2000). A positive correlation between ovary ecdysteroids levels and ovarian development has been demonstrated in the shore crab
Along with MIH, GIH/VIH is produced in the XO/SG complex (Van Herp and Soyez, 1997). In our study, ESA induced two physiological responses: the induction of 20E levels and the removal of GIH/VIH hormones. However, since both MIH and GIH/VIH are of the same CHH family type, their functions may be redundant. Hence, in some decapods, distinguishing MIH from GIH/VIH is problematic.
Eyestalk ablation may affect MF production. In an arthropod- specific second step, FA is converted to MF by P450 monooxygenase and farnesoic acid O-methyltransferase (FAMeT) (Goldstein and Brown, 1990; Holford et al., 2004). Crustacean FAMeT catalyzes the methylation of FA to MF; cDNAs for this protein have been isolated from various crustacean species, including lobsters, shrimp, and crabs, and its expression is induced by ESA (Gunawardene et al., 2002; Holford et al., 2004; Kuballa et al., 2007). Eyestalk ablation in spider crabs
In conclusion, the high-dose MF treatment (1.0 μg) stimulated molting, growth, and ovarian maturation in