The aquaculture industry is playing an increasingly important role in meeting the demand for fish for human consumption. The rapid development of the aquaculture industry is the most pronounced in Asia, which contributes about 89% of the global aquaculture production (Karunasagar, 2012). This increase in aquaculture production has resulted in an increased dependency on use of formulated diets for fish. Feeds used in aquaculture must be nutritionally suitable, but a great emphasis is being placed on the cost of feedstuffs as they account for about 50% of the total farm production costs for most aquatic species (Coyle et al., 2004). Due to the deficiency and expenditure of imported ingredients such as fish meal, soybean meal, and wheat flour, identifying alternative ingredients for use in markets that import much of their grain is important. Several scientific studies have examined alternative protein sources to fish meal, but limited studies on alternative carbohydrate sources have been published. Recently, because of the increasing expense and uncertain availability of many ingredients such as wheat flour (Rahman et al., 2013), we decided to investigate alternative carbohydrate sources. Replacement of wheat flour with less-expensive energy sources would be beneficial in reducing feed costs.
Distillers dried grain (DDG), a cereal by-product of the distillation process, is becoming an increasingly important ingredient due to its comparative affordability and rich vitamin and mineral contents (Cromwell et al., 1993). Traditionally, DDG has been utilized as a protein supplement (about 30% crude protein) in terrestrial animal feeds (De Godoy et al., 2009; Lim et al., 2009; Saunders and Rosentrater, 2009). DDG also has great potential to be used as a fish feed ingredient because of its relatively high protein content comparable to other grains used in fish feed production, and because it is very economically priced (Zhou et al., 2010; Li et al., 2011). Incorporation of DDG into the diet may be less expensive than other ingredients such as wheat flour (Rahman et al., 2013). Belyea et al. (2004) reported that this by-product of the drygrind, resulting from the yeast fermentation of cereal grains, is a valuable feed ingredient. Yamamoto et al. (2004) reported that fermentation is actually a cost-effective and suitable technique for drying out moist products, with minimal nutrient decline. Moreover, the fermentation of plant ingredients was demonstrated to induce substantial inactivation of antinutritional factors (Reddy and Pierson, 1994), improve the nutritional quality (Canella et al., 1984), increase the apparent digestibility (Kiers et al., 2000), and extend the shelf life of the resulting product (Skrede and Nes, 1988). DDG has been shown to be a viable potential substitute protein and/or energy source pertaining to aquafeed (Chevanan et al., 2010). Several studies have demonstrated that corn-based DDG is a suitable aquaculture dietary component on the basis of the growth performance of fish such as rainbow trout (Cheng and Hardy, 2004), channel catfish (Webster et al., 1993; Li and Robinson, 2007; Lim et al., 2009), and tilapia (Wu et al., 1997; Coyle et al., 2004; Lim et al., 2007; Schaeffer et al., 2009).
Rockfish
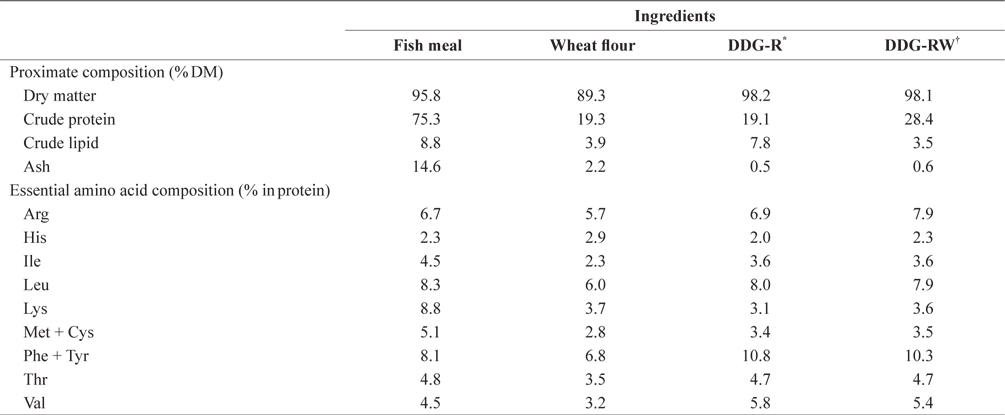
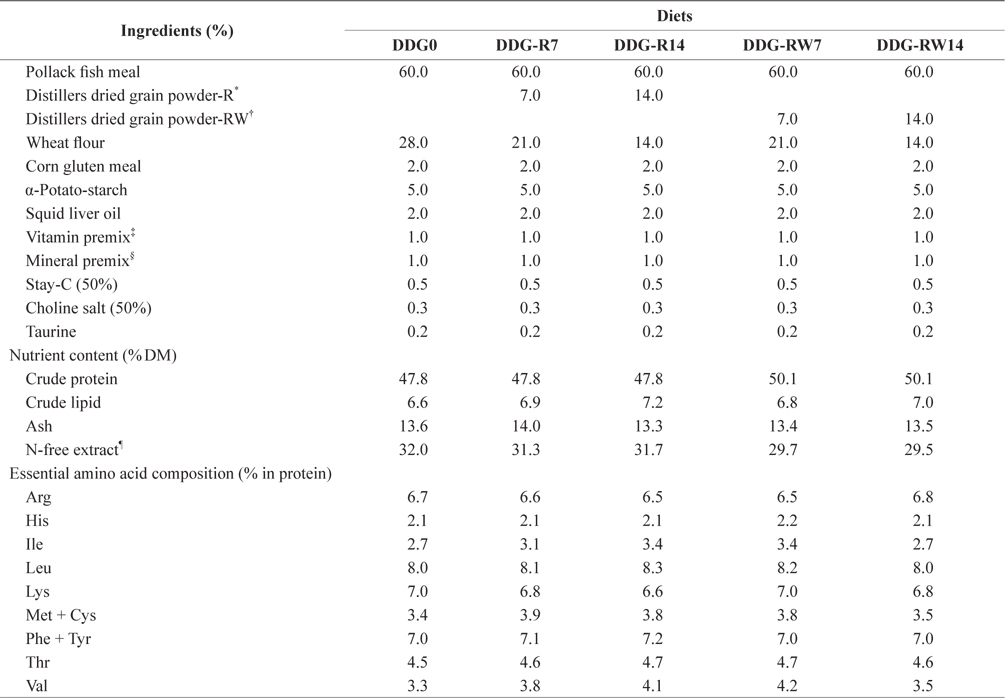
Proximate and essential amino acid compositions of the ingredients used in the experimental diets are shown in Table 1. Ingredients and chemical compositions of the experimental diets are presented in Table 2. Five isonitrogenous and isocaloric diets were formulated to contain 0% DDG (DDG0), 7% and 14% DDG from rice (diets DDG-R7 and DDG-R14, respectively), as well as 7% and 14% DDG from a mixture of rice and wheat flour (diets DDG-RW7 and DDG-RW14, respectively). Pollack fish meal was used as the primary protein source. Fish oil was used as the lipid source. DDG was sourced as a by-product of Makgeolli (a traditional alcoholic beverage native to Korea) production, and was produced by filtration of an aqueous mixture of fermented rice and/or wheat flour with
[Table 1.] Chemical composition of ingredients used in the experimental diets
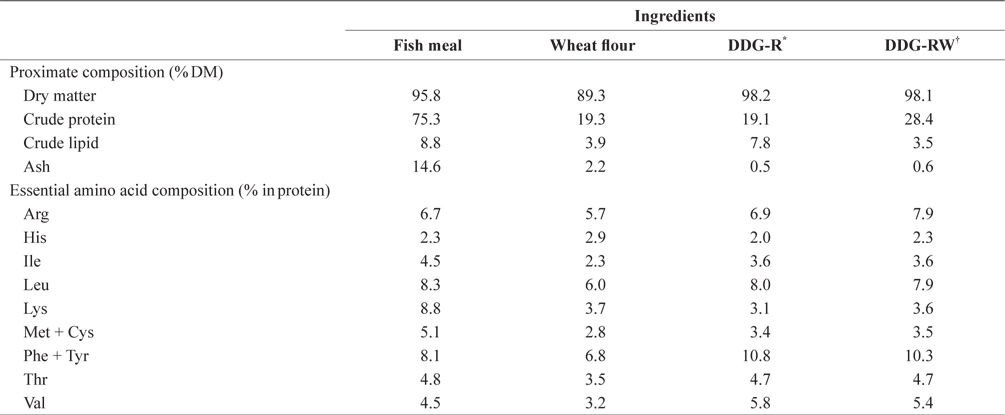
Chemical composition of ingredients used in the experimental diets
[Table 2.] Ingredients and chemical composition of the experimental diets
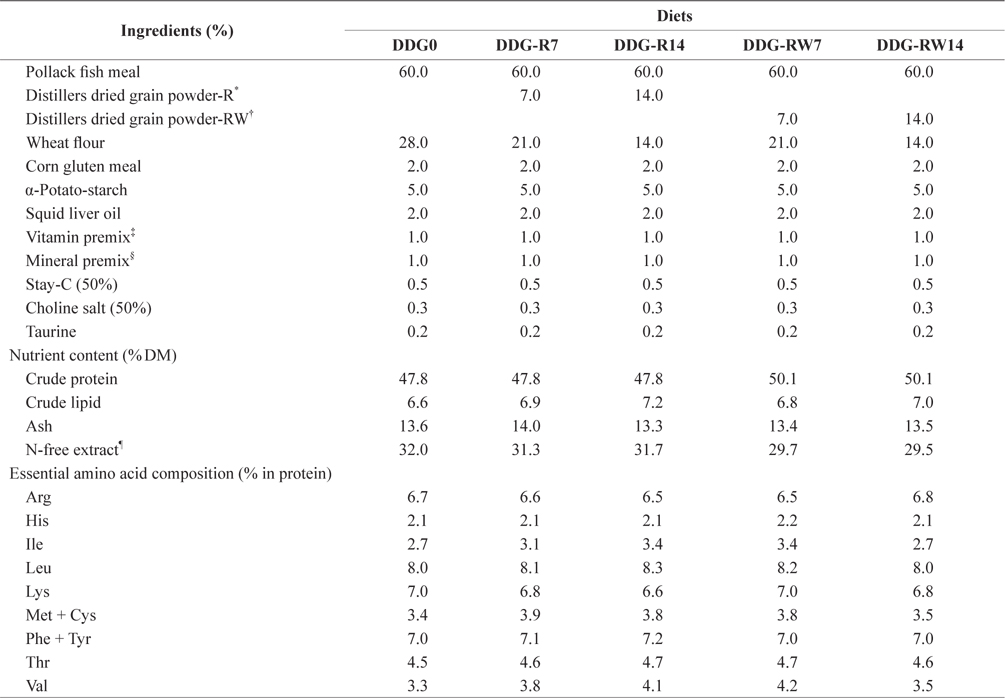
Ingredients and chemical composition of the experimental diets
>
Experimental fish and feeding conditions
Juvenile rockfish were transported from a private hatchery (Namhae, Korea) to the Marine Biology Center for Research and Education at Gangneung-Wonju National University (Gangneung, Korea) and were acclimated to laboratory conditions by feeding with commercial pellets for 2 weeks before starting the feeding trial. After the acclimation period, 450 juvenile rockfish (mean body weight, 68.0 ± 0.4 g) were randomly distributed into 15 400-L cylindrical plastic tanks (300 L water volume) at a density of 30 fish per tank. Each experimental diet was fed to three groups of fish to visual satiation twice per day (09:00 and 17:00 h) for 9 weeks. Filtered seawater was supplied at a flow rate of 4 L/min in each tank, and the mean water temperature and salinity were 20.4 ± 2.0℃ and 34 ± 0.1 ppt, respectively. The natural photoperiod was maintained during the feeding trail. Records were kept of daily feed consumption, mortalities, and feeding behavior.
>
Sample collections and analytical methods
At the end of each feeding experiment, all of the fish in each tank were collectively weighed after anesthetizing with tricaine methanesulfonate (MS222; Sigma, St. Louis, MO, USA) at a concentration of 100 mg/L after starvation for 24 h. At the end of the feeding trials, five fish per tank were sampled and stored at –25℃ for the analysis of proximate and essential amino acid composition. Proximate composition was analyzed according to standard methods (Association of Official Analytical Chemists, 1995). Crude protein was determined by the Kjeldahl method using an auto Kjeldahl System (Buchi, Flawil, Switzerland). Crude lipid was analyzed with ether extraction in a Soxhlet extractor (SER 148; VELP Scientifica, Milano, Italy). Moisture was determined using a drying oven at 105℃ for 6 h, and the ash content was determined after combustion at 550℃ for 4 h in a muffle furnace. Amino acid compositions of the experimental diets and whole body of fish were analyzed with acid hydrolysis with 6 N HCL (reflux for 23 h at 110℃) using an automatic amino acids analyzer (Hitachi, Tokyo, Japan).
Blood samples were taken from the caudal veins of five fish per tank using heparinized syringes. Plasma was collected after centrifugation at 7,500
>
Radical scavenging activities
At the end of the feeding trials, five fish per tank were sampled and stored at –75℃ for antioxidant activity analysis. Samples were extracted from plasma and liver, and were homogenized (Wiggenhauser, Berlin, Germany) with extract buffer in 5 mM Tris-HCl and 35 mM glycine (pH 8.4) followed by centrifugation (13,000 g for 10 min at 4℃). The supernatant was then collected and analyzed for its radical scavenging activity.
Evaluation of 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging activity was performed using the method described by Nanjo et al. (1995). A 30-μL peptide solution (or ethanol itself, as the control) was added to 30 μL of DPPH (60 μmol/L) in ethanol solution. After mixing vigorously for 10 s, the solution was transferred to a 100-μL quartz capillary tube, and the scavenging activity of the peptides on DPPH radicals was determined using a spectrometer (JEOL Ltd., Tokyo, Japan). After 2 min, the spin adduct was determined on an electron spin resonance (ESR) spectrometer. The measurement conditions were as follows: magnetic field, 336.5 ± 5 mT; power, 5 mW; modulation frequency, 9.41 GHz; amplitude, 1 × 1,000; and sweep time, 30 s.
Hydroxyl radicals were generated by an iron-catalyzed Fenton Haber–Weiss reaction and reacted rapidly with electron spin trap 5,5-dimethyl-1-pyrroline-
Alkyl radicals were generated by 2, 2-azobis-(2-amidinopropane)-hydrochloride (AAPH). The phosphate-buffered saline (pH 7.4) reaction mixtures included 10 mmol/L AAPH, 10 mmol/L 4-POBN, and known concentrations of the sample (100 μg/mL). The mixtures were incubated at 37℃ in a water bath for 30 min (Hiramoto et al., 1993), then transferred to a capillary tube. The spin adduct was recorded using a spectrometer (JEOL Ltd.). The measurement conditions were as follows: modulation frequency, 100 kHz; microwave power, 10 mW; microwave frequency, 9,441 MHz; magnetic field, 336.5 ± 5 mT; and sweep time, 30 s.
DPPH, hydroxyl, and alkyl radical scavenging activities (RSA) were computed according to the following equation in which
The data were subjected to one-way analysis of variance (ANOVA) using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Significant differences (
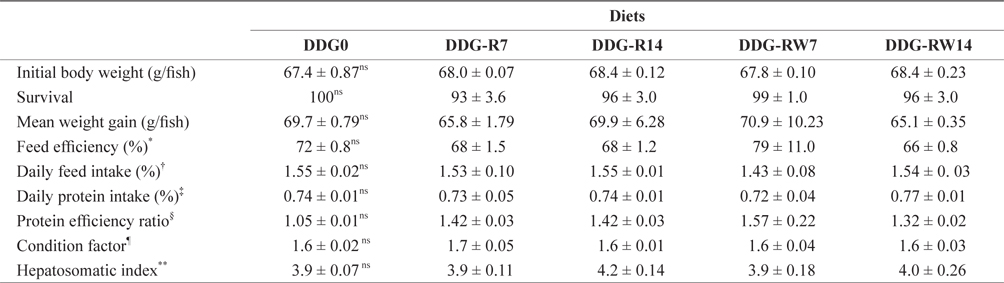
Growth performance, feed utilization, and morphological parameters of juvenile rockfish fed the experimental diets containing DDG are presented in Table 3. Survival, weight gain, feed efficiency, daily feed intake, daily protein intake, and protein efficiency ratio of fish were not affected by dietary DDG additions (
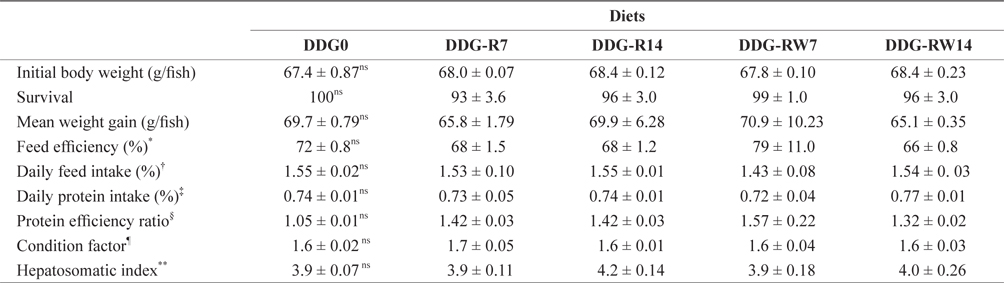
Growth performance, feed utilization and morphological parameters of juvenile rockfish fed the experimental diets for 9 weeks
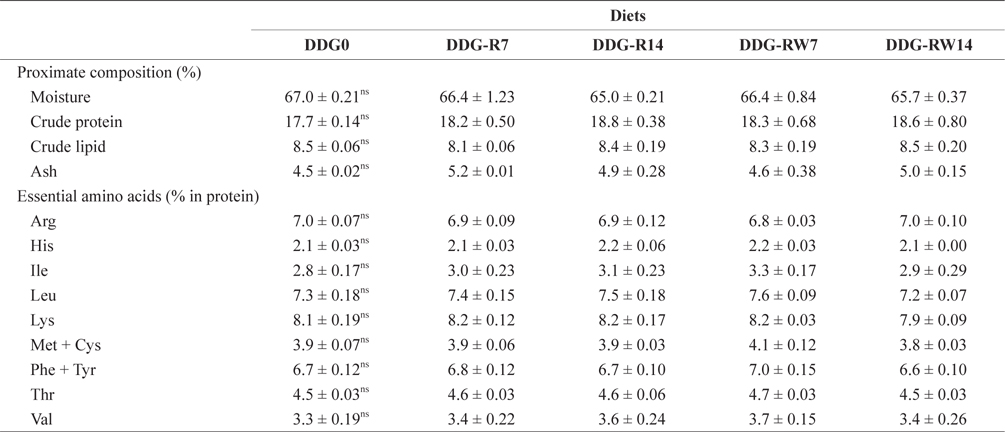
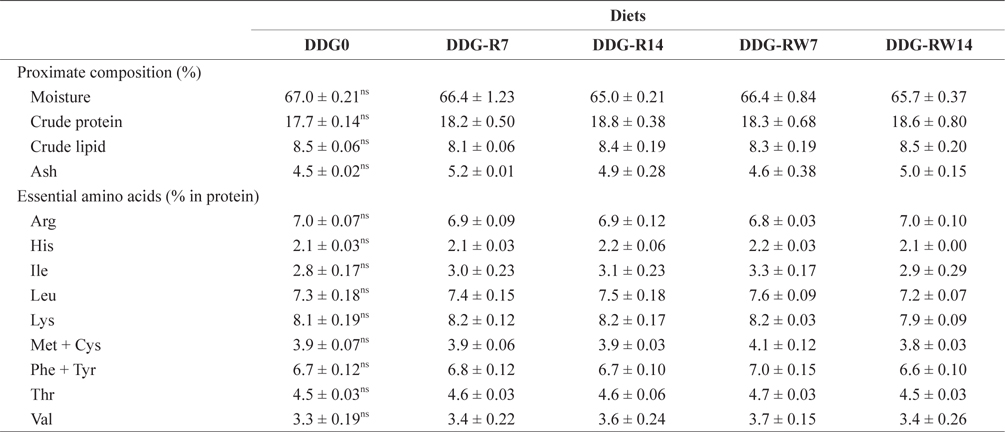
Proximate and essential amino acid composition of the whole body in juvenile rockfish fed the experimental diets for 9 weeks
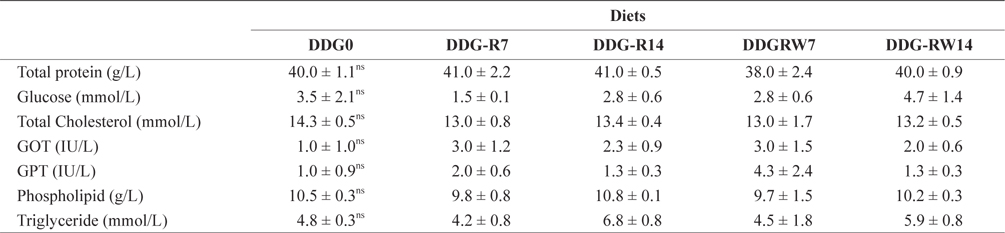
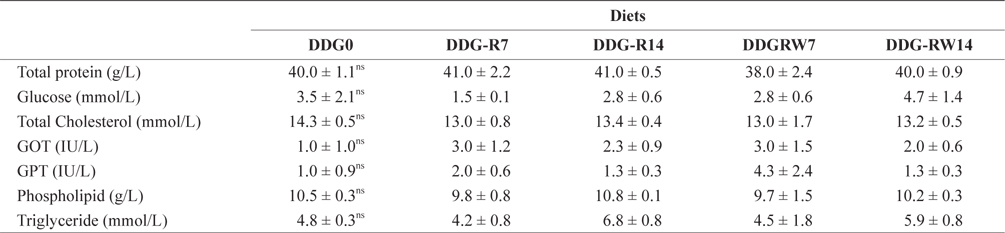
Hematological parameters of the plasma of juvenile rockfish fed the experimental diets for 9 weeks
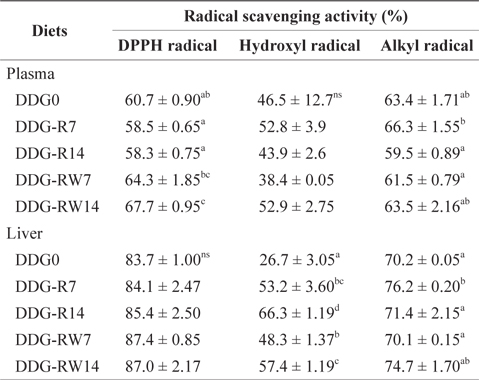
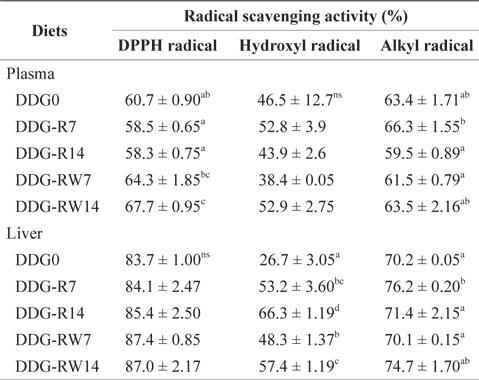
Radical scavenging activity of the plasma and liver of juvenile rockfish fed the experimental diets for 9 weeks
The results of the present study demonstrate that the inclusion of DDG from rice and/or wheat flour, at a rate of up to 14% DDG, is appropriate for the diet for juvenile rockfish as a partial replacement for wheat flour without reducing growth performance. Li et al. (2010) observed that inclusion of corn-based DDG in the diet of channel catfish improved weight gain as well as the feed efficiency ratio compared to an all plant-protein diet (control). Several scientific studies have demonstrated that relatively high levels of corn-based DDG could be used in channel catfish diets without adversely minimizing fish growth performance (Robinson and Li, 2008). Previous studies have shown that corn-based DDG could be used to partially replace soybean meal in channel catfish diets (Webster et al., 1993). Lim et al. (2007) found that up to 20% corn-based DDG could be included in the diet of Nile tilapia
Lim et al. (2007) reported that different levels of corn-based DDG did not significantly influence the hematological parameters of the Nile tilapia. Similar results were obtained for the hematological parameters of juvenile rockfish in the present study. Rockfish fed diets containing DDG did not significantly differ among the treatments in terms of the body proximate composition. Earlier studies with Nile tilapia (Li et al., 2011) and rainbow trout (Barnes et al., 2012) demonstrated that whole-body proximate composition was not significantly affected by dietary levels of DDG. The successful inclusion of DDG in the diet of juvenile rockfish is probably associated with the nutritional components that arise during fermentation by
The antioxidant activity of an extract can be specified more accurately by assessing the scavenging activities on DPPH, hydroxyl, and alkyl radicals, using an ESR spin-trapping technique. A positive correlation has been reported between free radical scavenging activity and total phenolic compounds (Siriwardhana et al., 2003; Heo et al., 2005). Velioglu et al. (1998) reported that a highly significant relationship existed between total phenols and antioxidant activity for methanolic extracts of fruits, vegetables, and grain products. DPPH is a stable free radical, which accepts an electron or hydrogen radical to become a stable diamagnetic molecule. DPPH has been widely used to examine the free radical scavenging effect of various natural plants and vegetable extracts as antioxidants (Yen and Chen, 1995; Matsukawa et al., 1997; Yamaguchi et al., 1998). To our knowledge, no information is available on the scavenging activities of DDG as a feed ingredient for fish. Kim and Lee (2008) reported that DPPH free radical scavenging activity increased gradually with an increase in dietary
The results of this experiment suggest that DDG is a suitable ingredient to replace wheat flour. DGG could be used at a rate of up to 14% in the diet without incurring any negative effects on growth performance, while reducing the cost of feed formulation for juvenile rockfish.